Aortic Dissection
What is Aortic Dissection?
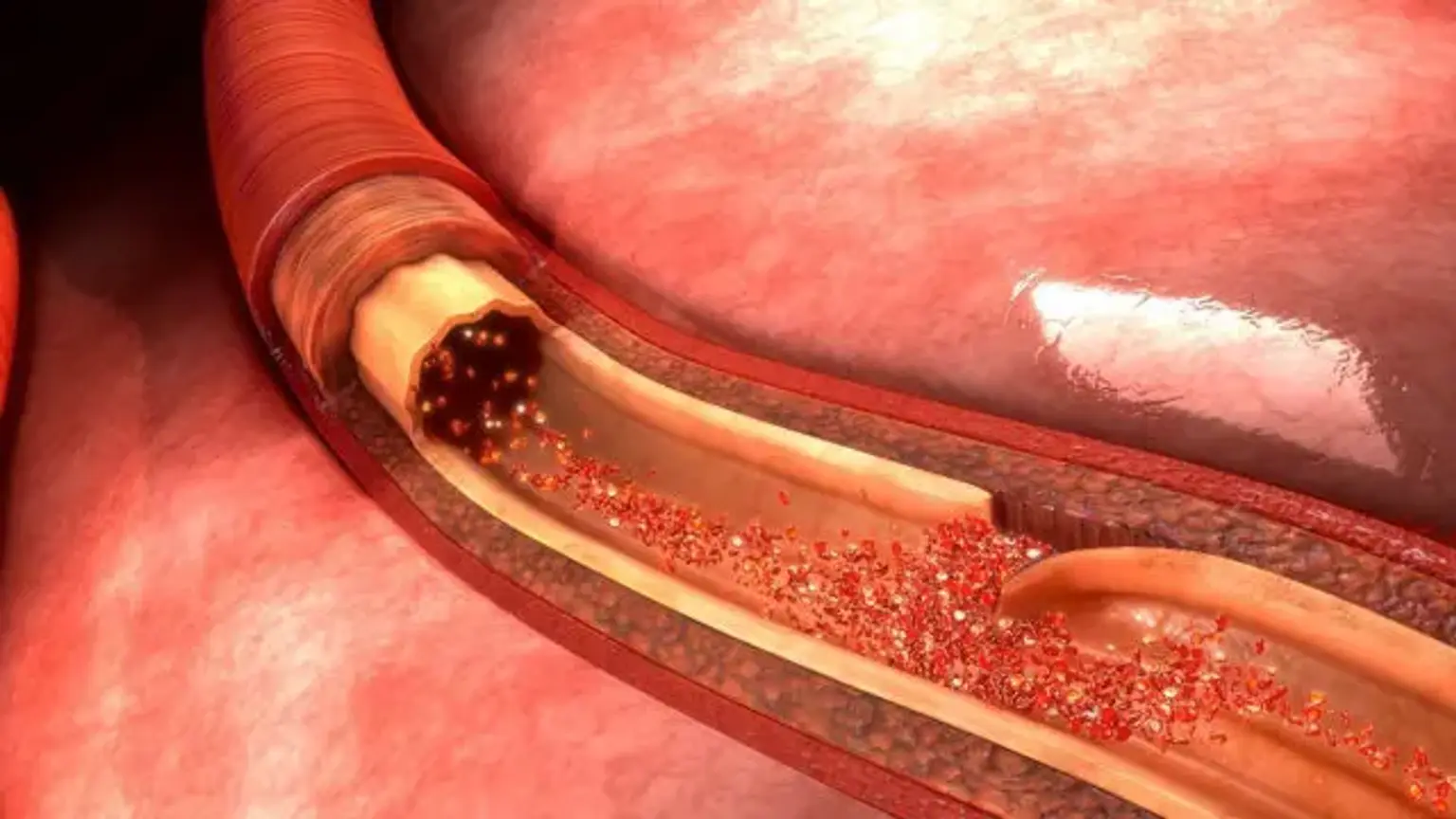
Acute aortic dissection (AAD) is an uncommon but deadly condition. Aorta dissection is caused by the separation of the aortic wall's layers. A rupture in the intimal layer causes the dissection to advance (either proximal or retrogradely), owing to blood penetration between the intima and media. Aortic dissection is linked with a very high mortality rate; the majority of patients die before reaching the emergency department. Patients with a chronic aortic dissection (one that has been present for more than two weeks) had a somewhat better prognosis.
Acute aortic dissection (within 2 weeks) is associated with significant morbidity and death. The first 7 days have the greatest mortality rate; in fact, many patients die before they report to the emergency department (ED) or before a diagnosis is made in the ED. Patients with chronic aortic dissection (lasting more than two weeks) had a better prognosis. Despite advances in diagnostic and treatment techniques, the death rate linked with aortic dissection remains significant.