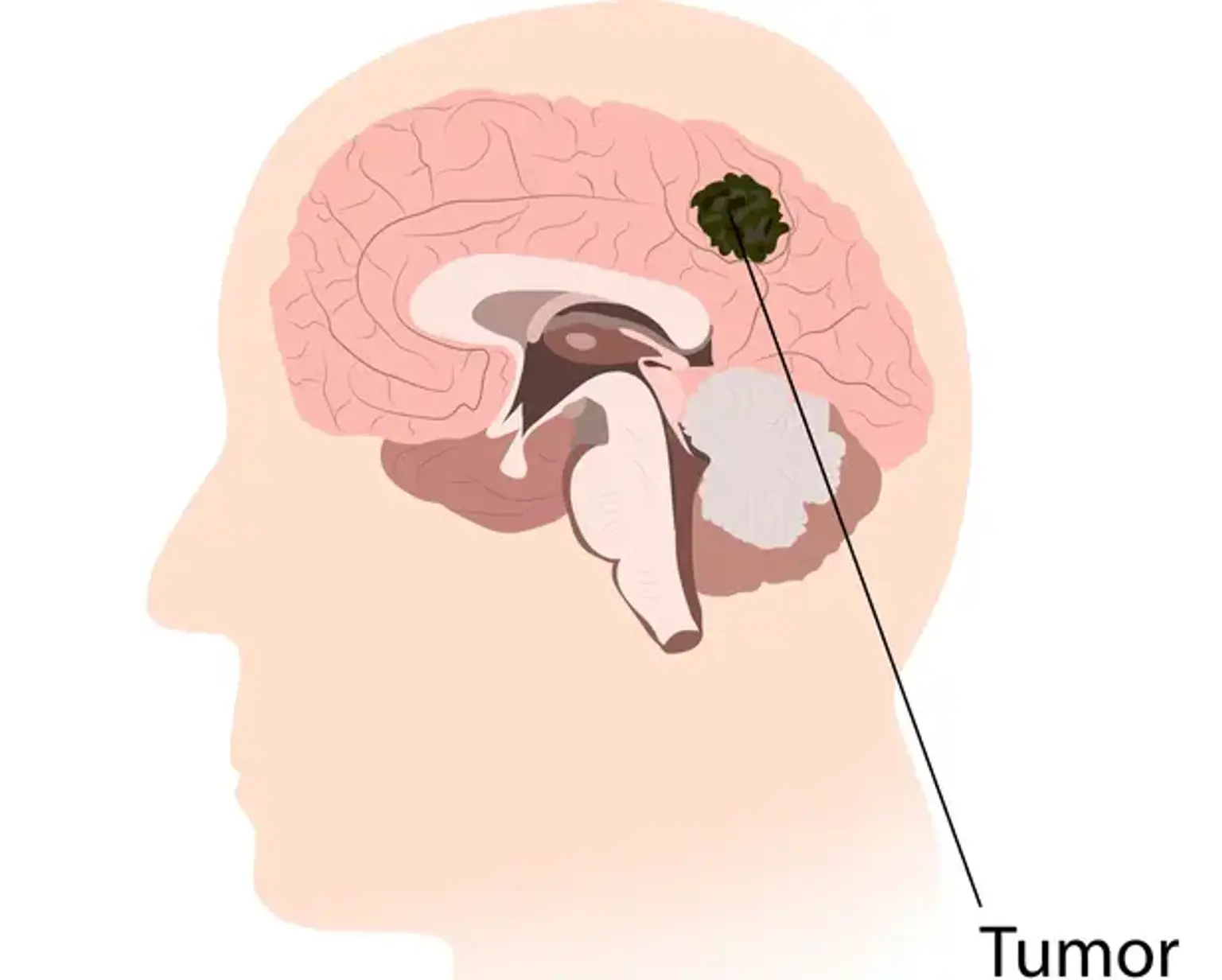
Astrocytoma
Overview
Astrocytoma develops from astrocytes, which are star-shaped glial cells in the cerebrum. It is the most frequent kind of glioma, mostly affecting the brain and occasionally the spinal cord. Glial tumors account for 60 percent of all brain tumors. They are a leading cause of death and morbidity in both young and old people. Astrocytomas are a kind of brain tumor that is rather prevalent. To avoid the significant morbidity and mortality associated with this disorder, they must be detected and treated as soon as possible.