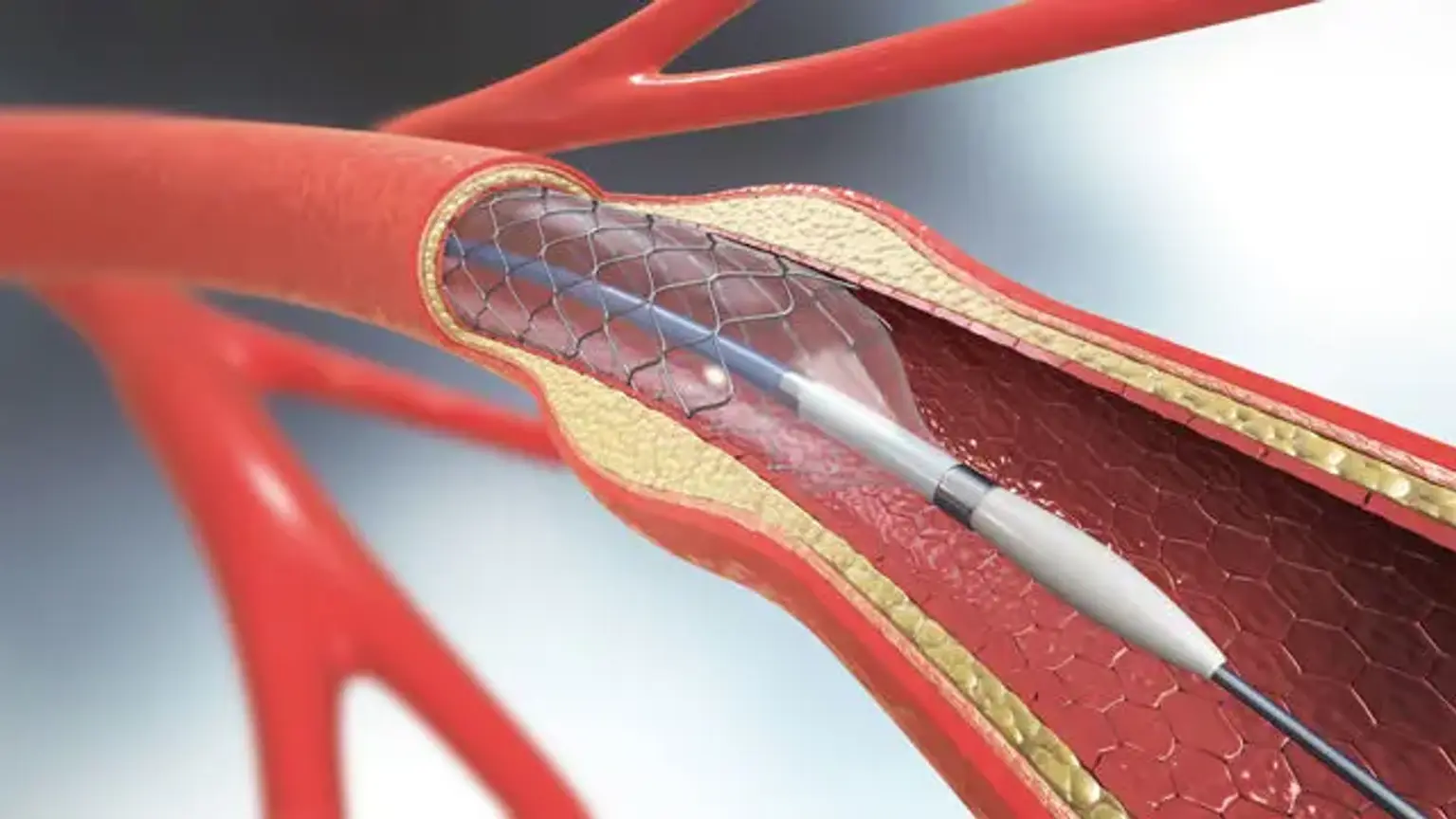
Carotid Artery Stenting
Traditional treatment for high-grade asymptomatic and symptomatic carotid artery stenosis has been carotid endarterectomy (CEA). Carotid endarterectomy is a procedure that involves exposing the carotid artery and removing plaque from the carotid bulb and internal carotid artery through a neck cut. Minimally invasive procedures have progressed in vascular surgery, as they have in many other surgical fields throughout time. Smaller cuts, less postoperative discomfort, a lower risk of postoperative wound problems, and a shorter hospital stay are all advantages of these procedures. One such procedure is carotid artery stenting (CAS), which can be done via a transfemoral or transcarotid route.
Anatomy and Physiology
The aortic arch (left) or the brachiocephalic trunk (right) give rise to the common carotid artery. It is divided into two carotid arteries: internal and external carotid. The external carotid artery supplies blood to the face, scalp, and neck, whereas the internal carotid artery supplies blood to the brain. At various points, the internal and external carotid arteries develop collaterals. In the event that either artery becomes clogged, this is useful for maintaining blood flow through collateral circulation. There are three distinct anatomic forms of the aortic arch. To make things easier, an imagined horizontal line is marked through the top of the aortic arch and imagined parallel lines are made to compare the origins of the great vessels emerging off the arch. All great vessels begin near the imaginary horizontal line drawn through the top of the aortic arch in a type 1 arch. A type 2 aortic arch is defined by the origins of all major vessels falling within the second parallel line. The sources of all major vessels lie within the third parallel line for a type 3 aortic arch. The angulation of the aortic arch, as it relates to the type of aortic arch, has a significant impact on CAS.