Crystal Arthropathies
Overview
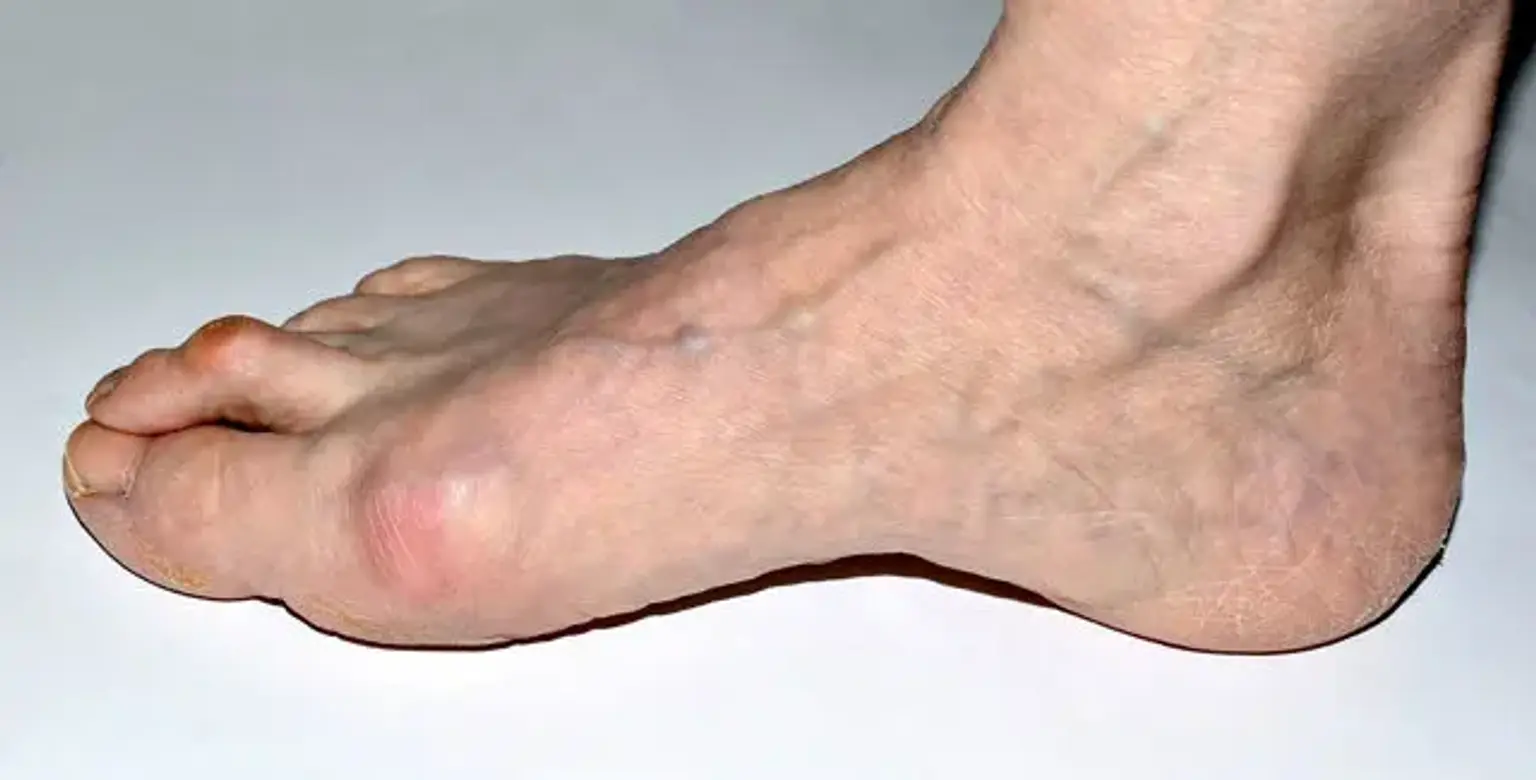
Crystal arthropathies are a set of joint illnesses characterized by crystal deposits in the joints and surrounding soft tissues. Gout and calcium pyrophosphate deposition are the two most prevalent kinds (CPPD). Crystalline arthropathies can cause joint degeneration and kidney illness in the long run.