Cytogenetic analysis of chorionic villi
Overview
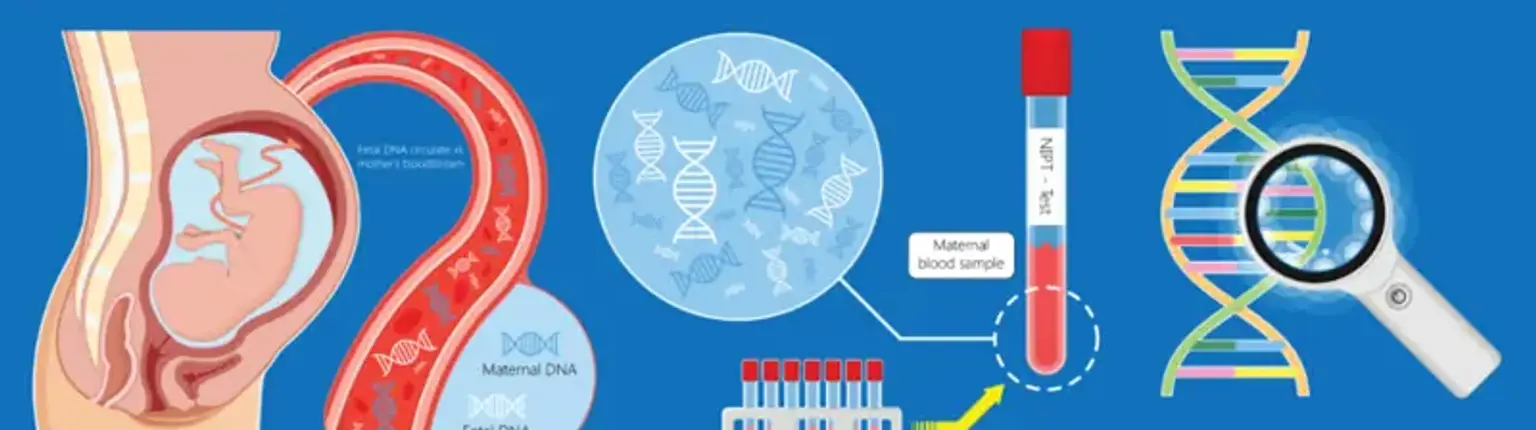
Prenatal diagnostics makes use of chorionic villus sample to discover fetal genetic disorders. Its benefits include the ability to do the operation during the first trimester of pregnancy, a reasonably quick outcome, and a risk of miscarriage equivalent to that of amniocentesis.