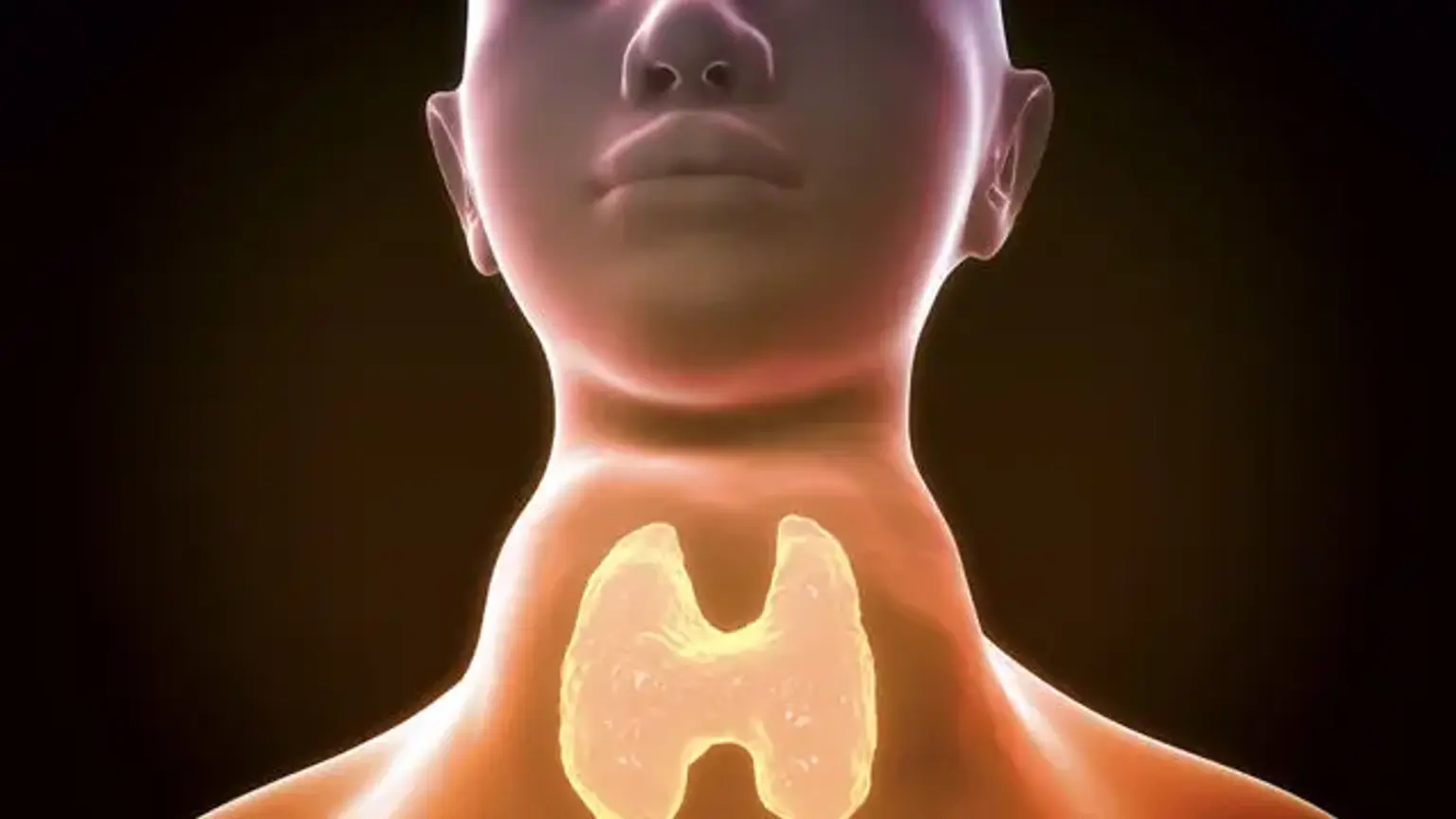
Diffuse Toxic Goiter (Graves' Disease)
Overview
Patients with goiter frequently present to outpatient clinics with a wide range of problems. Goiter has a wide range of causes and morbidities, and a correct diagnosis is critical for determining the best treatment strategy. Goiter, whether simple or nodular, harmless or poisonous, may have a significant influence on a patient's quality of life and well-being, as well as long-term physical and aesthetic health impacts.
Diffuse toxic goiter is an autoimmune disorder characterized by a diffusely hyperplastic thyroid gland and excessive thyroid hormone production. Graves disease, the most prevalent cause of hyperthyroidism, is distinguished by symptoms such as diffuse toxic goiter, oculopathy, and pretibial myxedema/acropachy. Diffuse toxic goiter is also found in various autoimmune thyroid disorders that induce hypothyroidism, the most prevalent of which being Hashimoto thyroiditis.