Endometrial Hyperplasia
Overview
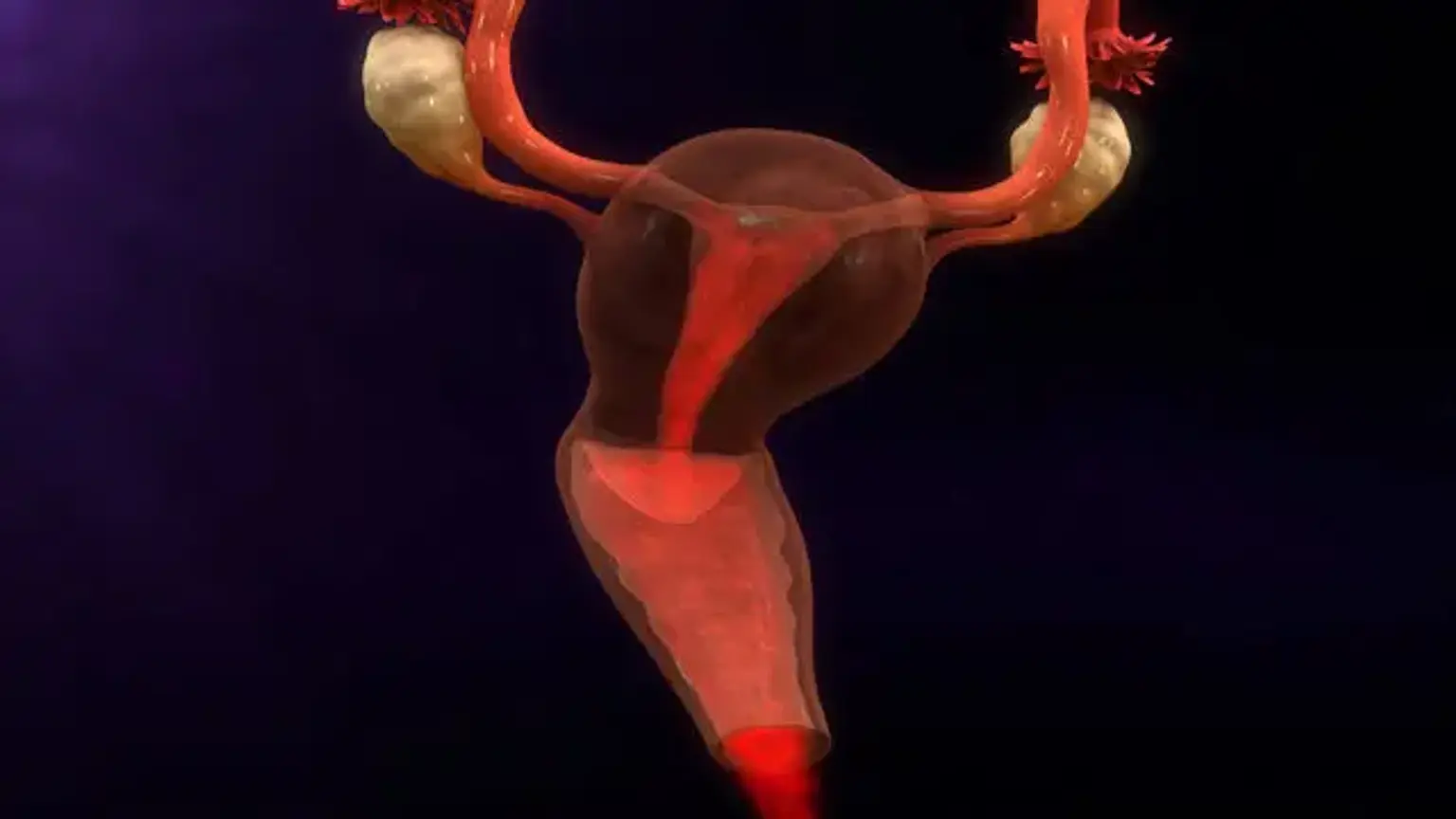
Endometrial hyperplasia occurs when the endometrium, or uterine lining, becomes too thick. Endometrial hyperplasia (EH) is a uterine disorder that encompasses a wide range of morphological endometrial changes. When compared to normal proliferative endometrium, it is primarily distinguished by an increase in the endometrial gland-to-stroma ratio.
The clinical relevance of EH stems from the likelihood of development to endometrioid endometrial cancer (EC), and 'atypical' types of EH are considered premalignant lesions. This disorder is not cancer, although it can progress to uterine cancer in some circumstances.