EUS-guided hepaticogastrostomy
Overview
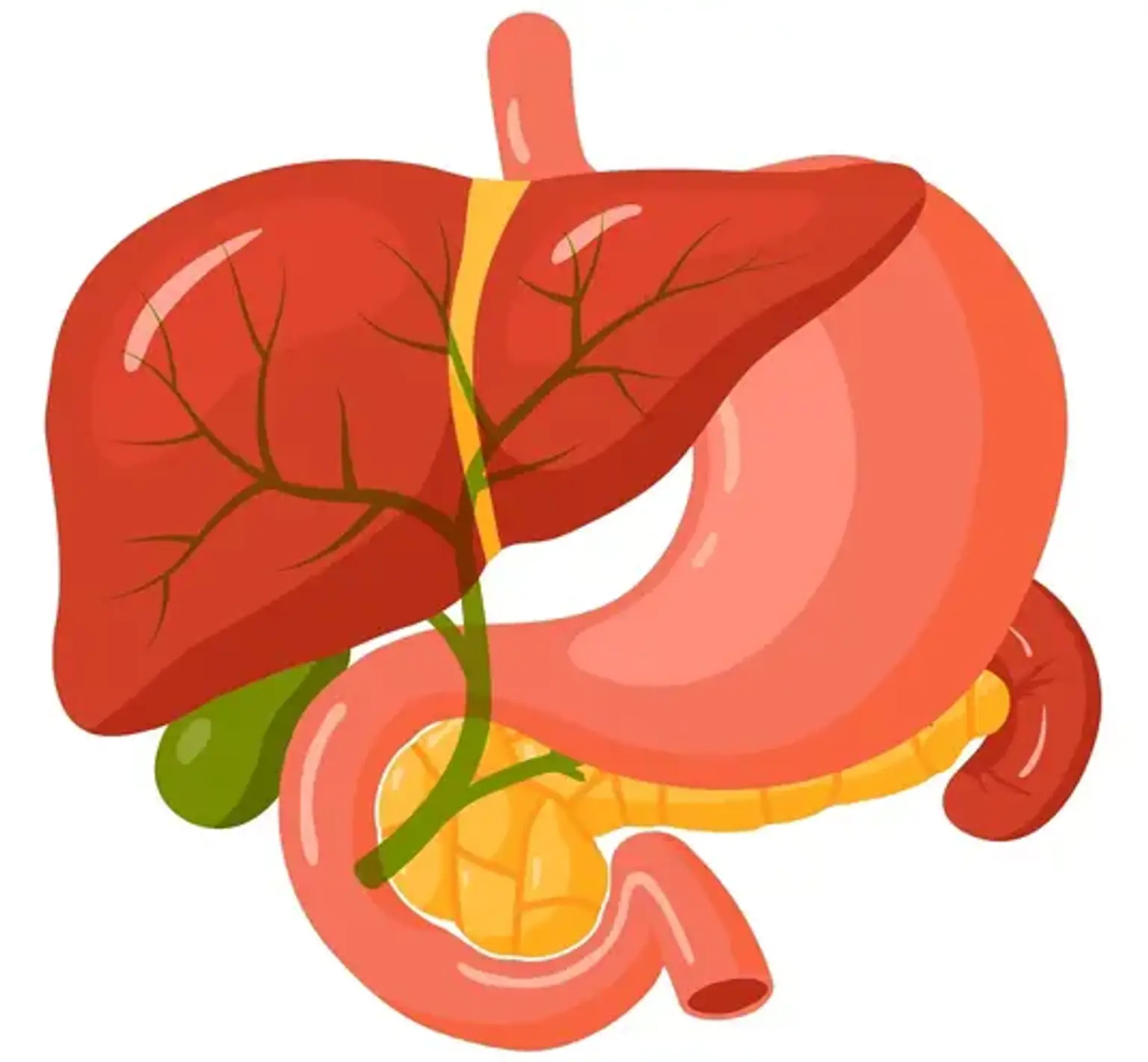
The liver produces and excretes bile. Bile travels through a network of tube-like structures known as bile ducts to the small intestine, where it aids in the digestion and absorption of food, and eventually out of the body via the digestive system. The liver, gallbladder, and pancreas are all connected to the small intestine through the common bile duct.
A biliary drain (also known as a biliary stent) is a thin, hollow, flexible tube with many tiny pores along one or both sides. When there is too much bile in the bile ducts, a biliary drain is employed. Bile can back up into the liver if the bile duct is blocked. Jaundice, a disorder in which the skin and whites of the eyes turn yellow, can result from this. A biliary drain aids bile passage from the liver into the gut when the bile duct is clogged. Depending on the kind of biliary tube, it may be attached to an external drainage bag.
Cholestasis is a condition in which the bile becomes clogged. Cholestasis can be caused by a number of conditions, including: