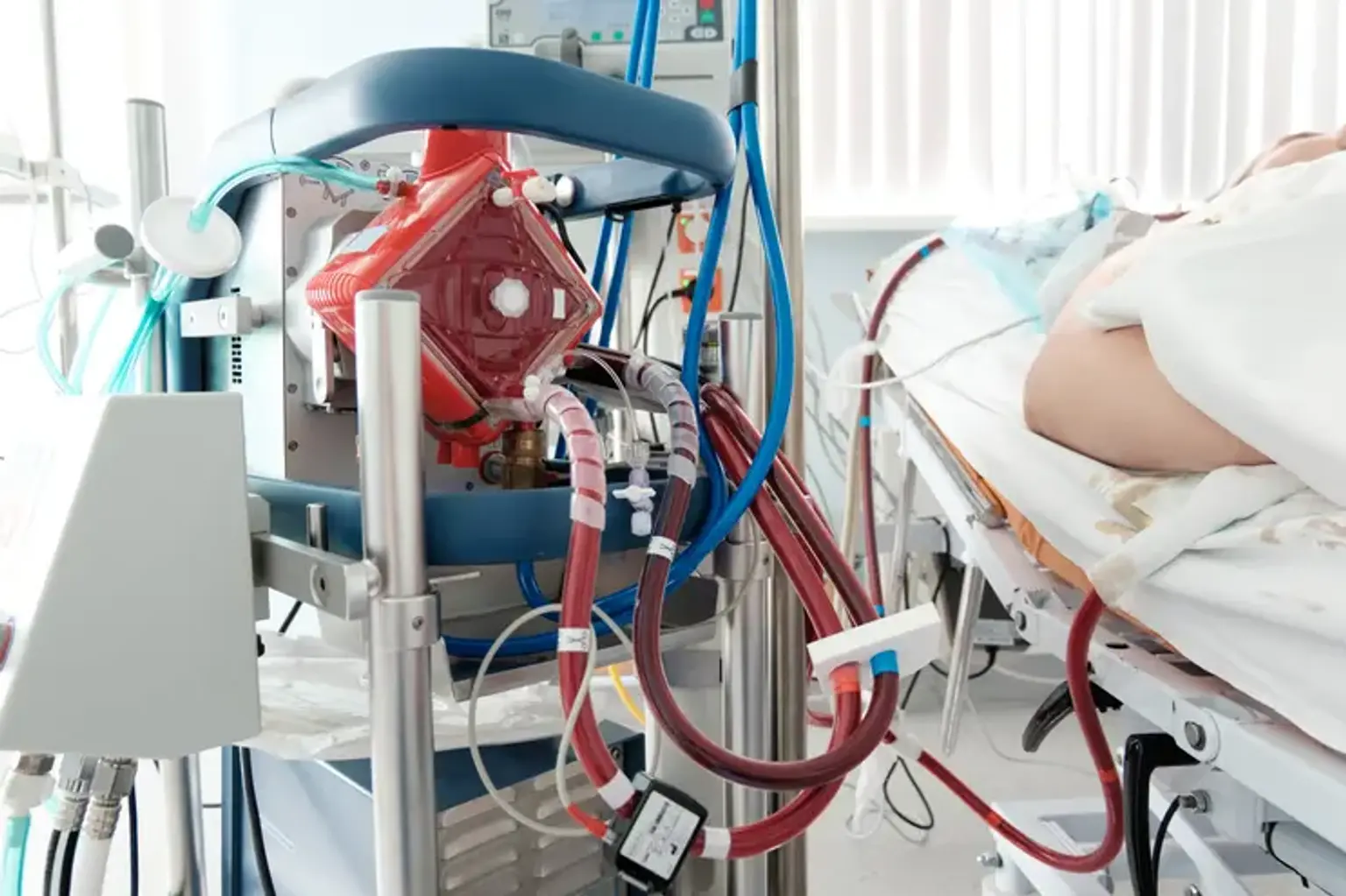
Extracorporeal membrane oxygenation (ECMO)
Overview
The heart and lungs are replaced by the Extracorporeal membrane oxygenation (ECMO) system. People who require ECMO assistance are cared for at a hospital's critical care unit (ICU). People are usually maintained by an ECMO machine for a few hours to days, but they may need it for a few weeks depending on how their disease improves. There are both similarities and variations in the use of ECMO in children and adults.