Familial hypercholesterolemia
Overview
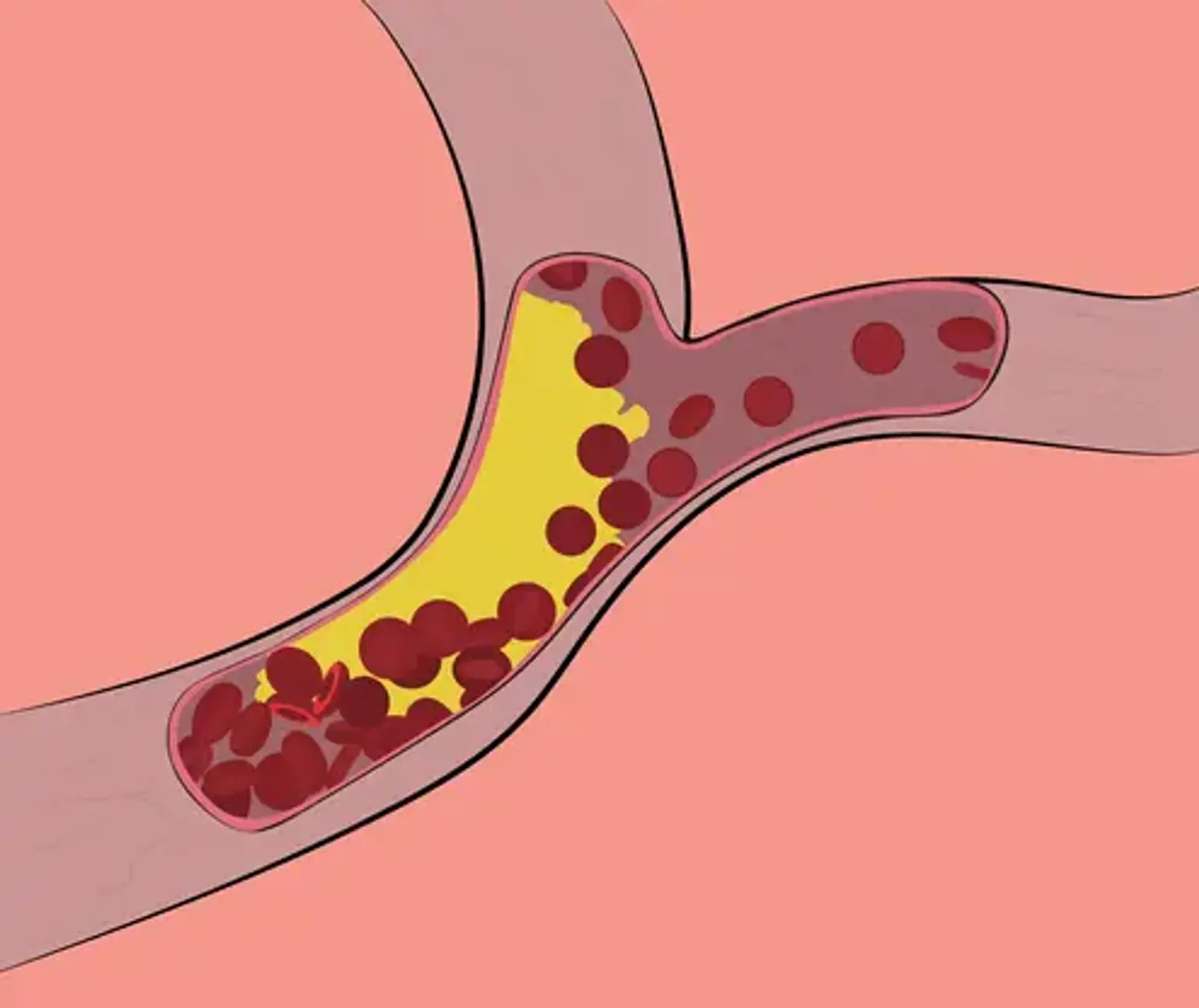
Familial hypercholesterolemia is a collection of inherited genetic abnormalities that cause a significant increase in blood cholesterol level. Although FH atherosclerosis develops largely in adulthood, it can begin as early as the first decade of life. The fact that early treatment of risk factors can reverse atherosclerotic alterations in the vascular system emphasizes the need of detecting and treating children with this disease as soon as possible.