Glioblastoma
Overview
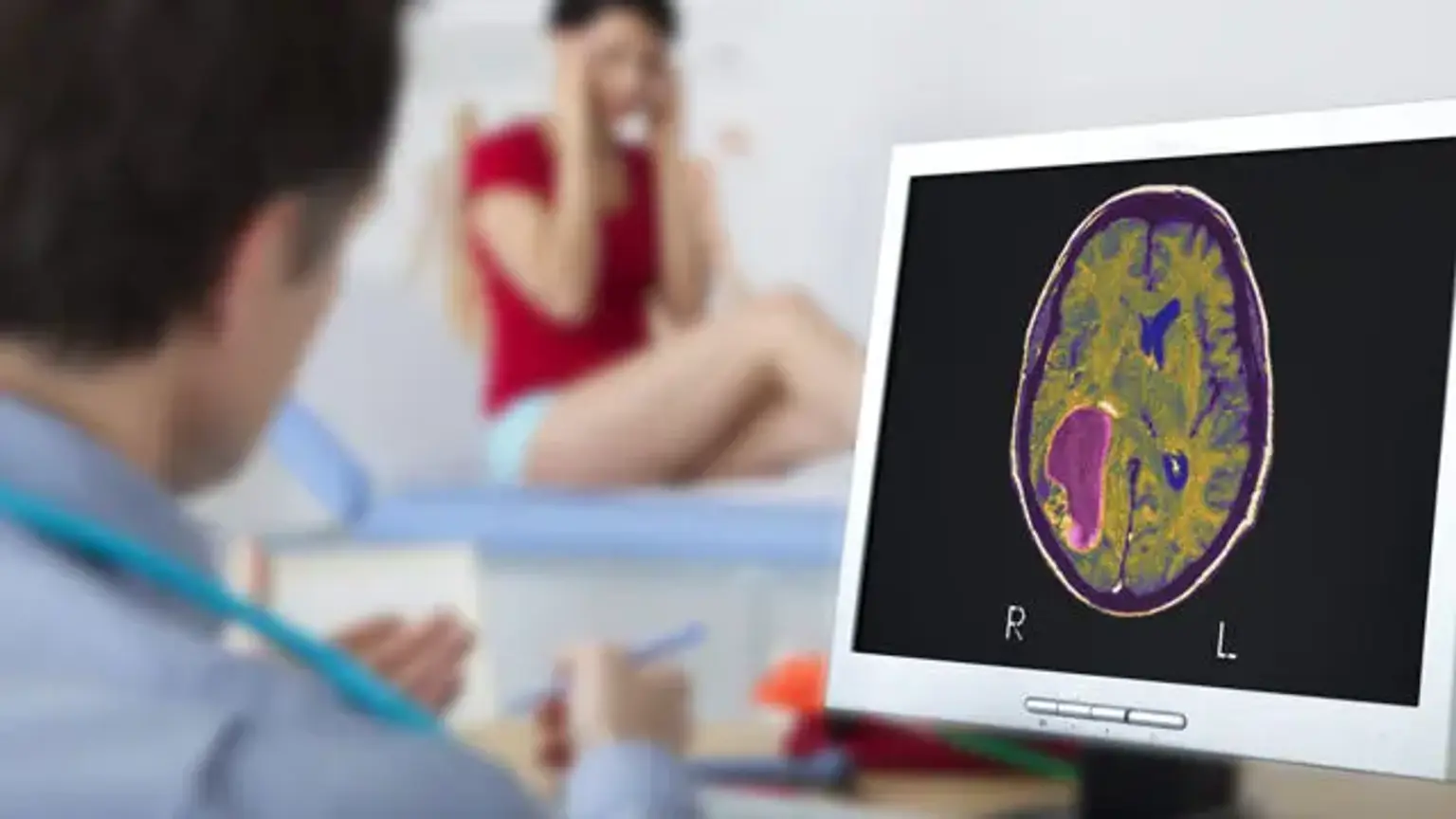
Glioblastoma, also known as glioblastoma multiforme (GBM), is the most malignant type of brain cancer. Glioblastoma symptoms and signs are initially nonspecific. They may include headaches, personality changes, nausea, and stroke-like symptoms. Symptoms frequently increase quickly and can lead to coma.
The majority of glioblastoma instances have no known cause. Genetic disorders such as neurofibromatosis and Li–Fraumeni syndrome, as well as past radiation therapy, are uncommon risk factors. Glioblastomas account for 15% of all brain tumors. They can arise from normal brain cells or from a pre-existing low-grade astrocytoma. A CT scan, an MRI scan, and a tissue biopsy are commonly used to make the diagnosis.
There is no known way to prevent the cancer. Surgery is usually the first step in treatment, followed by chemotherapy and radiation therapy. Temozolomide is a chemotherapeutic drug that is regularly used. To assist minimize swelling and discomfort, high-dose steroids may be utilized. Surgical excision of the tumor (decompression) is associated with enhanced survival, although only by a few months.