Glioma
Overview
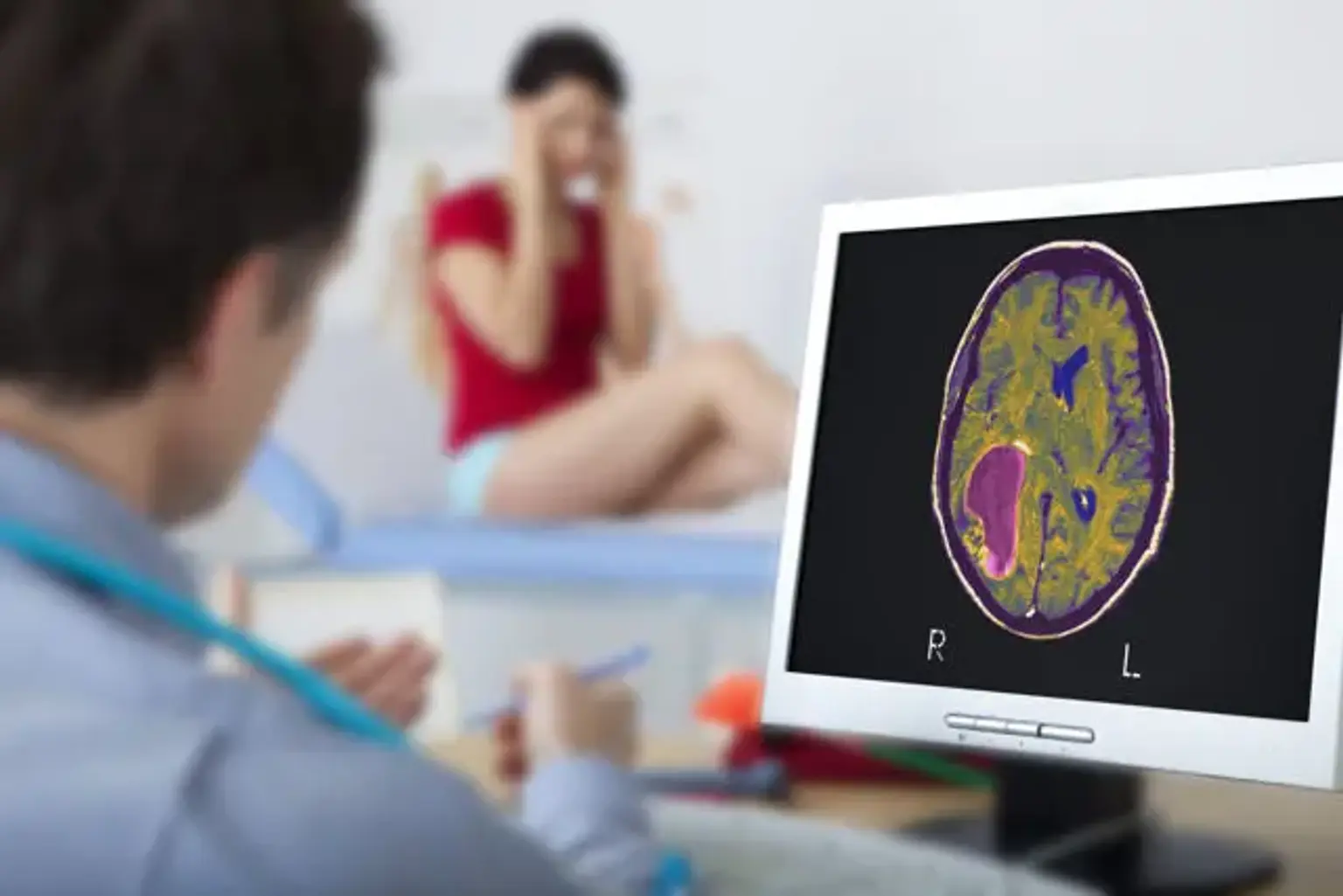
Glioma is the most prevalent kind of glial-cell-derived central nervous system (CNS) tumor. Every year, six cases of gliomas are identified per 100,000 persons in the United States. Gliomas are tumors that infiltrate the surrounding brain tissue in a highly diffuse manner. Glioblastoma is the most dangerous type brain tumor, whereas pilocytic astrocytomas are the least dangerous.
Previously, diffuse gliomas were divided into subgroups and grades based on histopathology, such as diffuse astrocytomas, oligodendrogliomas, or mixed gliomas/oligoastrocytomas.
Gliomas were recently classified using molecular and genetic markers. These advancements have more specific prognostic and therapeutic effects for glioma patients. Gliomas are graded from I to IV depending on the degree of proliferation indicated by the mitotic index and the presence or absence of necrosis, in addition to molecular and genetic markers.