Introduction
Hemangiomas are benign vascular tumors that commonly appear in infancy, often as a red or bluish raised lesion on the skin. These growths result from an abnormal proliferation of blood vessels and typically go through a cycle of rapid growth followed by gradual regression. While many hemangiomas resolve without intervention, some require medical treatment due to complications such as ulceration, obstruction of vital functions, or cosmetic concerns.
Early diagnosis and appropriate treatment are crucial to managing hemangiomas effectively. In some cases, hemangiomas can cause significant functional impairment, especially when located near the eyes, nose, mouth, or airways. Understanding the different types of hemangiomas, available treatment options, and potential complications can help caregivers and medical professionals make informed decisions about management strategies.
Hemangiomas are the most common benign tumors in infants, affecting approximately 4-5% of newborns. They are more prevalent in premature infants, females, and Caucasian populations. Although most hemangiomas are harmless, a small percentage require medical intervention due to their size, location, or growth pattern.
Types of Hemangiomas
There are two primary types of hemangiomas: infantile hemangiomas and congenital hemangiomas.
Infantile Hemangiomas: These are the most common type and typically appear within the first few weeks of life. They undergo a rapid proliferative phase (growth phase) for the first 6-12 months, followed by a gradual involution phase over several years.
Congenital Hemangiomas: Unlike infantile hemangiomas, these are fully developed at birth and do not go through a proliferative phase. They can be categorized as:
Rapidly Involuting Congenital Hemangiomas (RICH) – These shrink significantly within the first year of life.
Non-Involuting Congenital Hemangiomas (NICH) – These persist without shrinking and may require treatment.
Additionally, hemangiomas can be classified based on their depth:
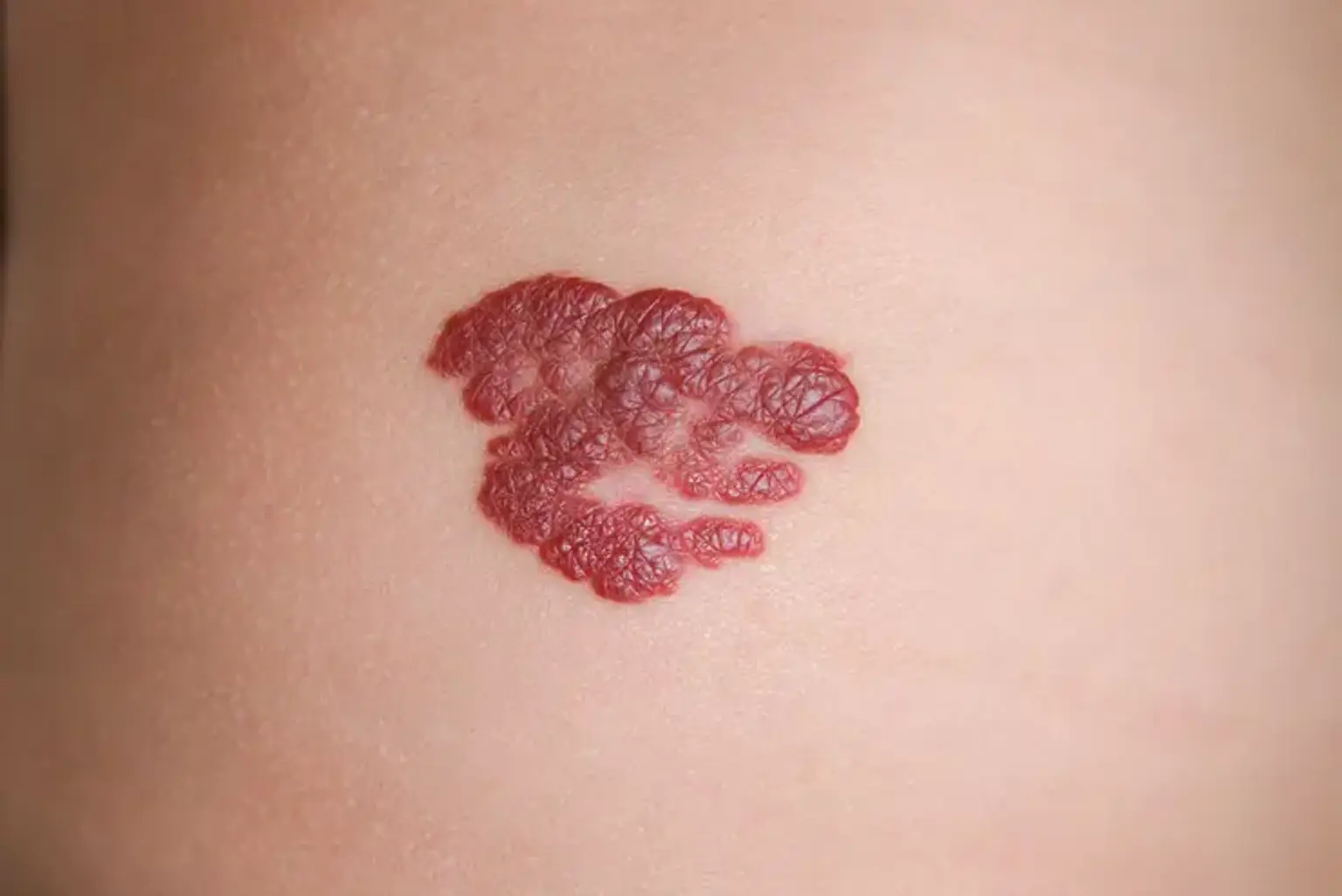
Superficial Hemangiomas: Appear as bright red, raised lesions on the skin’s surface (also called "strawberry hemangiomas").
Deep Hemangiomas: Develop beneath the skin and may have a bluish or skin-colored appearance.
Mixed Hemangiomas: Contain both superficial and deep components.