Hemostasis
Overview
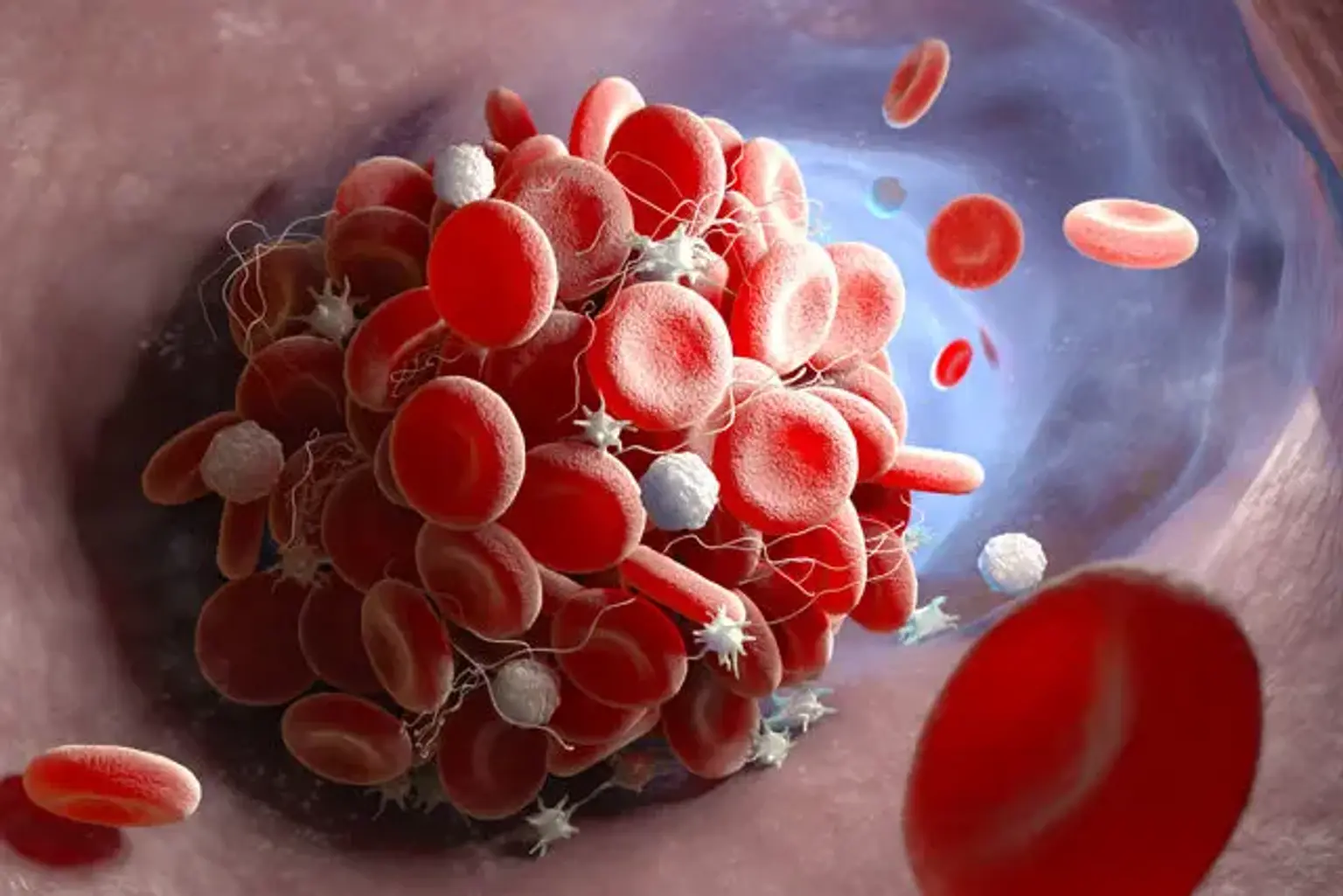
Hemostasis is the process that results in the cessation of bleeding from a blood vessel. It is a multi-step procedure with several interconnected steps. This cascade results in the development of a "plug" that seals the injured region of the blood vessel, thereby regulating the bleeding. It all starts with harm to the blood vessel lining.