Hyperthyroidism
Overview
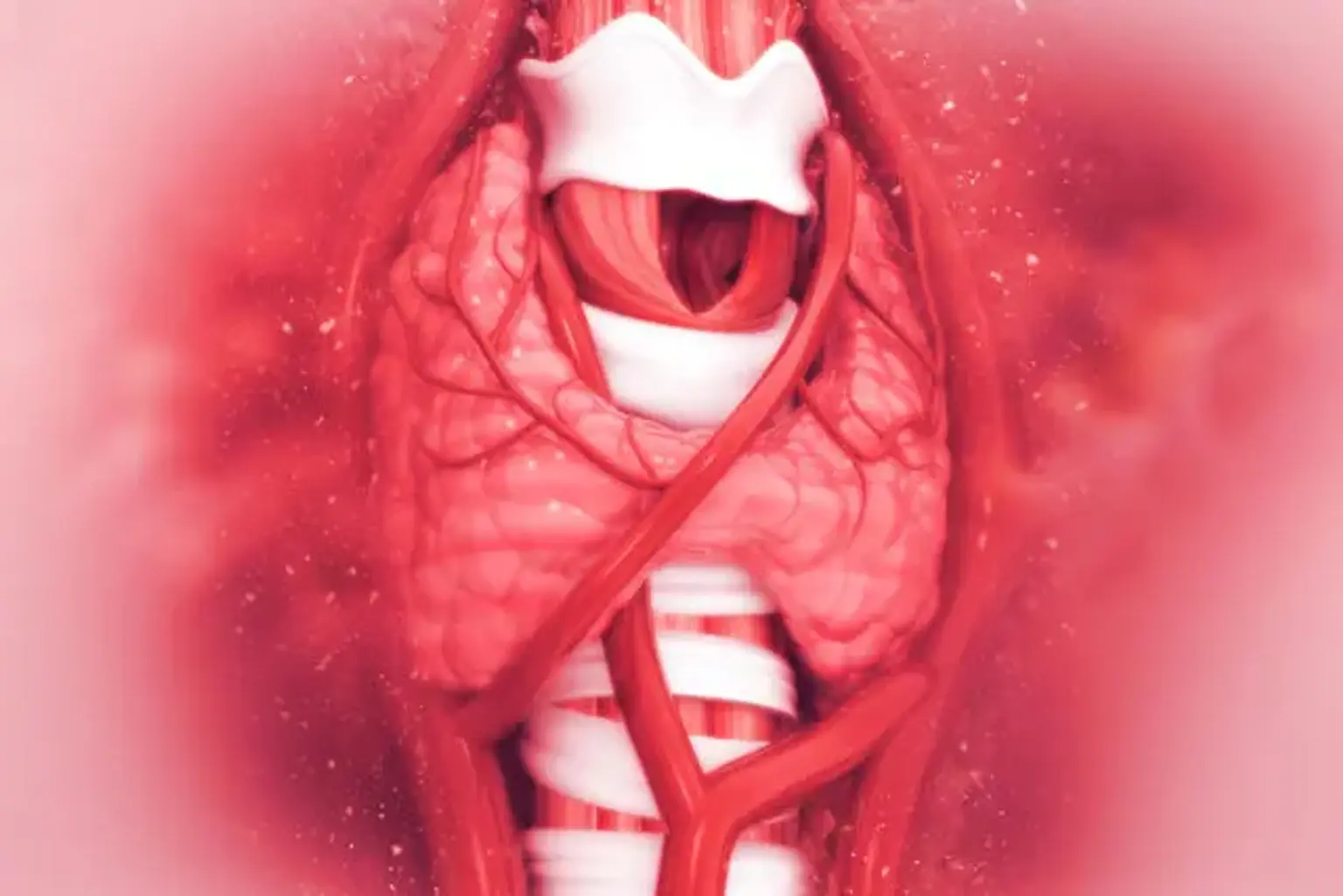
The thyroid gland is a bilobed organ found between the cricoid cartilage and the suprasternal notch in the front side of the trachea. A thyroid isthmus joins each lobe of the thyroid. The superior thyroid artery, which branches from the external carotid artery, and the inferior thyroid artery, a branch of the thyrocervical trunk, supply it. The word "hyperthyroidism" refers to a condition characterized by excessive thyroid hormone production.
It is a prevalent misperception that the terms thyrotoxicosis and hyperthyroidism are interchangeable. Excess thyroid hormone exposure to tissues is referred to as "thyrotoxicosis." Although hyperthyroidism and thyrotoxicosis are related and can be used interchangeably, it is vital to distinguish between the two.