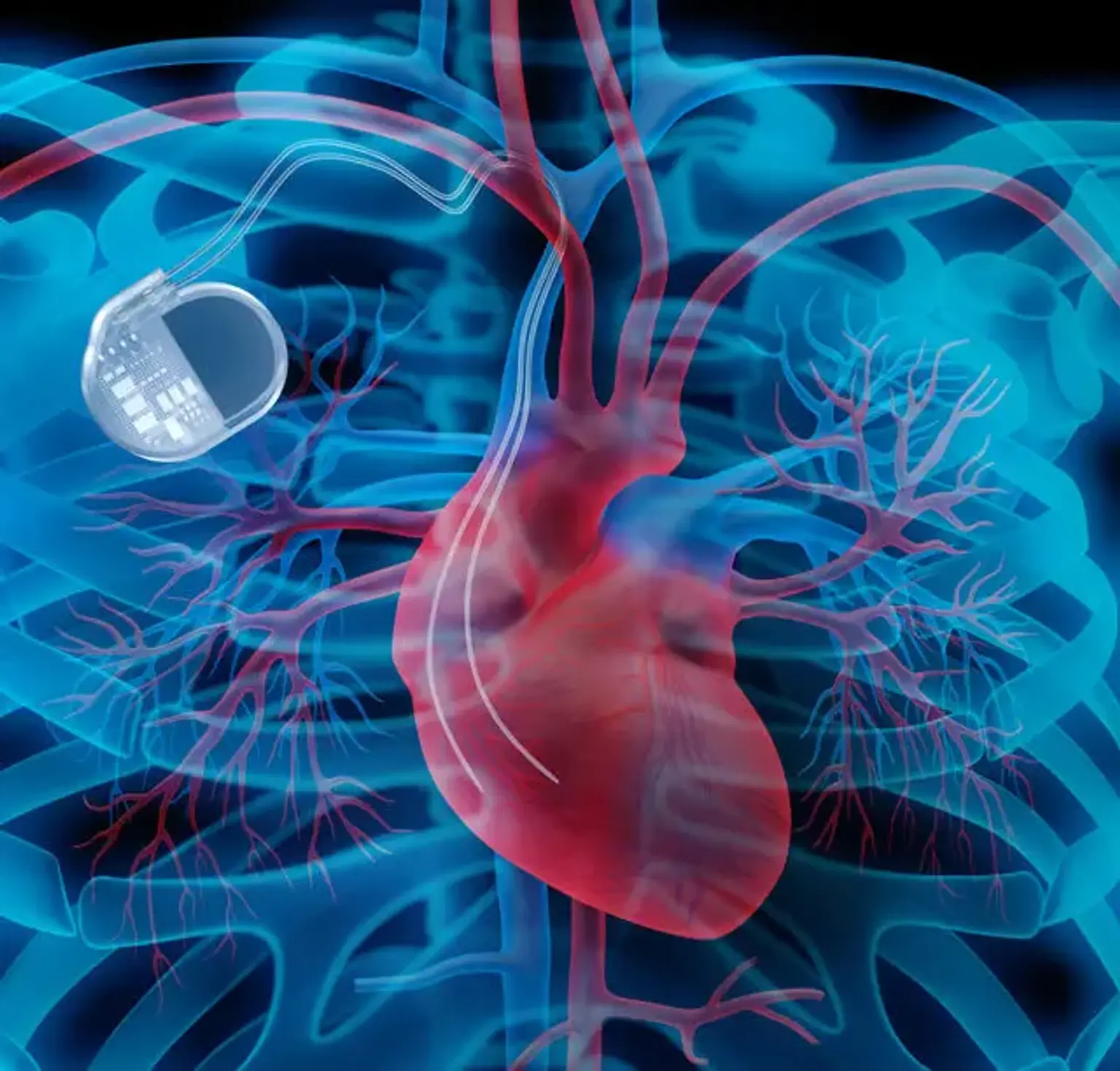
Pacemaker Implantation
Overview
A pacemaker (PM) is a medical device that regulates the heart's beating by using electrical impulses given by electrodes contracting the heart muscles. The basic function of this device is to maintain an adequate heart rate, either because the heart's natural PM is insufficient or because there is a block in the electrical conduction system.
Modern pacemakers are externally programmable, allowing the cardiologist to choose the best pacing settings for particular patients. Some implanted devices combine a PM with a defibrillator. PMs can be either transitory or long-term.
Temporary PMs are used to treat short-term cardiac disorders including a sluggish heartbeat induced by a heart attack, heart surgery, or a medication overdose. Permanent PMs are used to treat chronic cardiac rhythm disorders. Some arrhythmia symptoms, such as exhaustion and fainting, can be relieved by a PM. A PM can also assist a person with abnormal HRs in resuming a more active lifestyle.