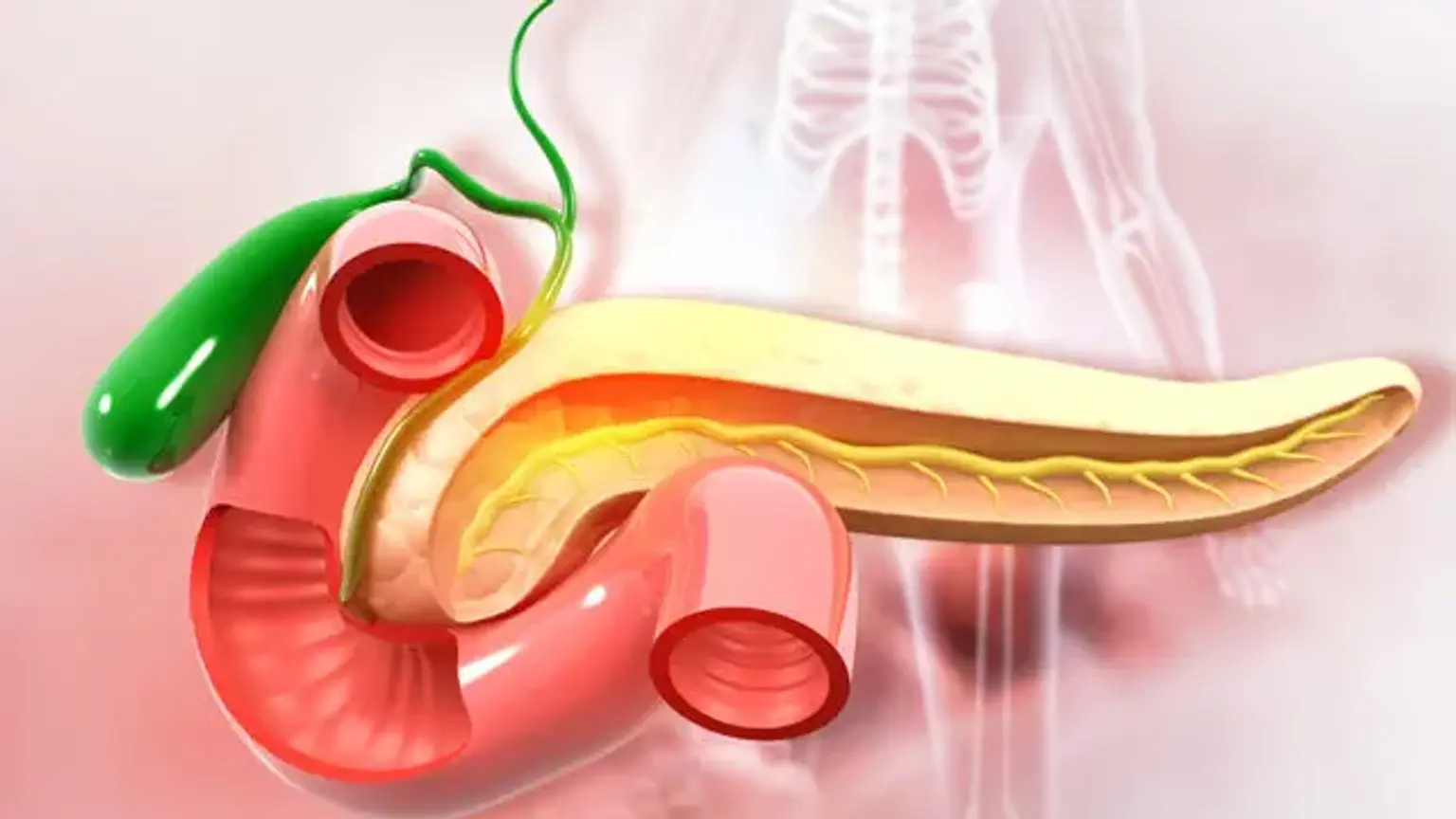
Pancreaticoduodenectomy
Overview
Despite advances in medical treatment, chemotherapy, radiation, and molecular biology, pancreatic cancer is the fourth leading cause of cancer mortality, with a 5-year survival rate of just approximately 12%. The pancreatoduodenectomy, often known as the Whipple surgery, is the surgical method of choice for pancreatic ductal adenocarcinomas that are resectable or borderline resectable.
Because organs in the proximal gastrointestinal system share a blood supply, surgical removal of the head of the pancreas entails removal of the duodenum, proximal jejunum, gallbladder, and, in certain cases, a portion of the stomach.