Pulmonary embolism (PE)
Overview
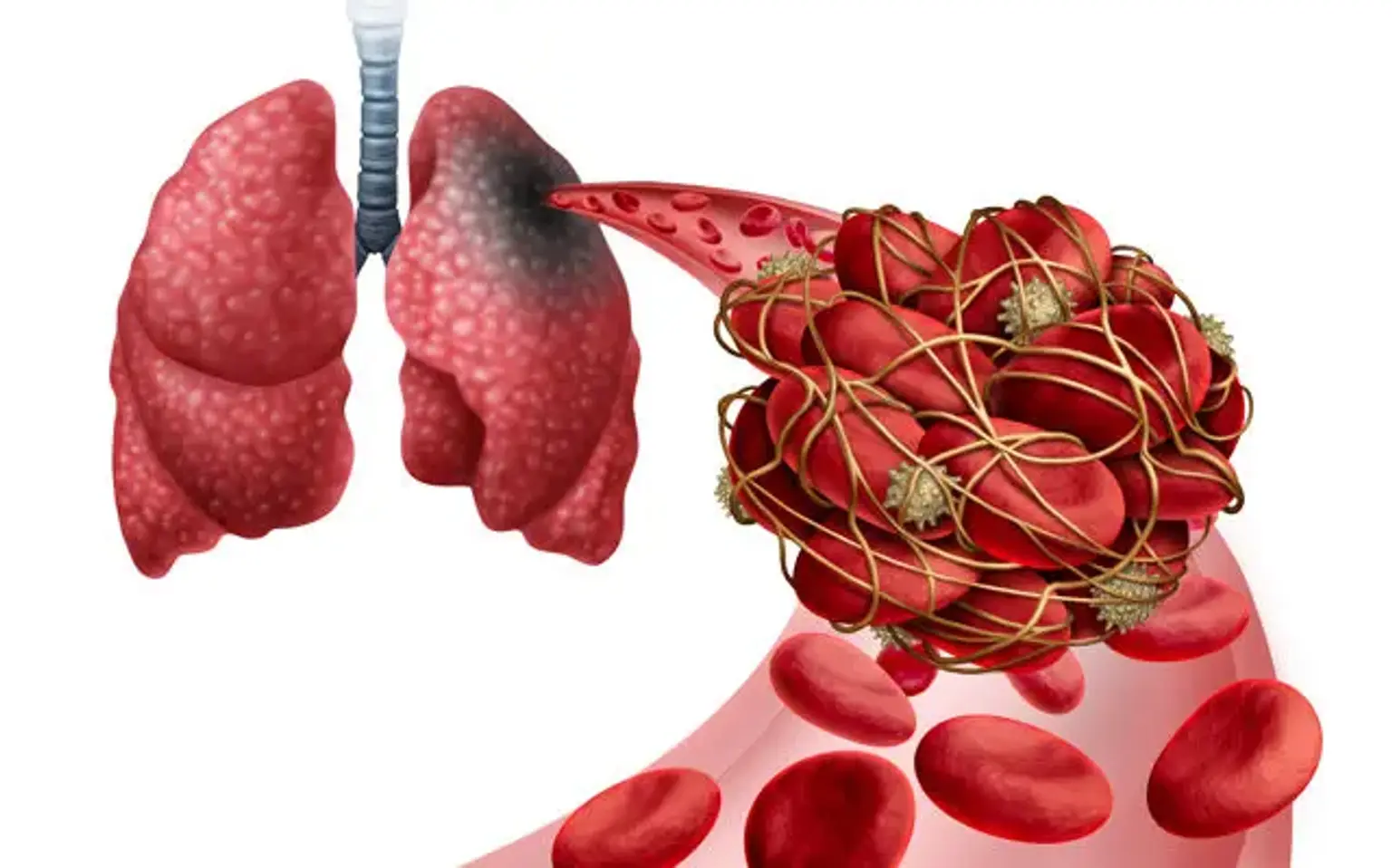
When a thrombus from someplace else disrupts the flow of blood in the pulmonary artery or its branches, this is referred to as pulmonary embolism (PE). A thrombus forms within the deep veins, most often in the lower limbs, in deep vein thrombosis (DVT). PE is often caused when a portion of the thrombus breaks off and enters the pulmonary circulation.
PE can develop very infrequently as a result of embolization of various materials into the pulmonary circulation, such as air, fat, or tumor cells. The combined spectrum of PE and DVT is known as venous thromboembolism (VTE).