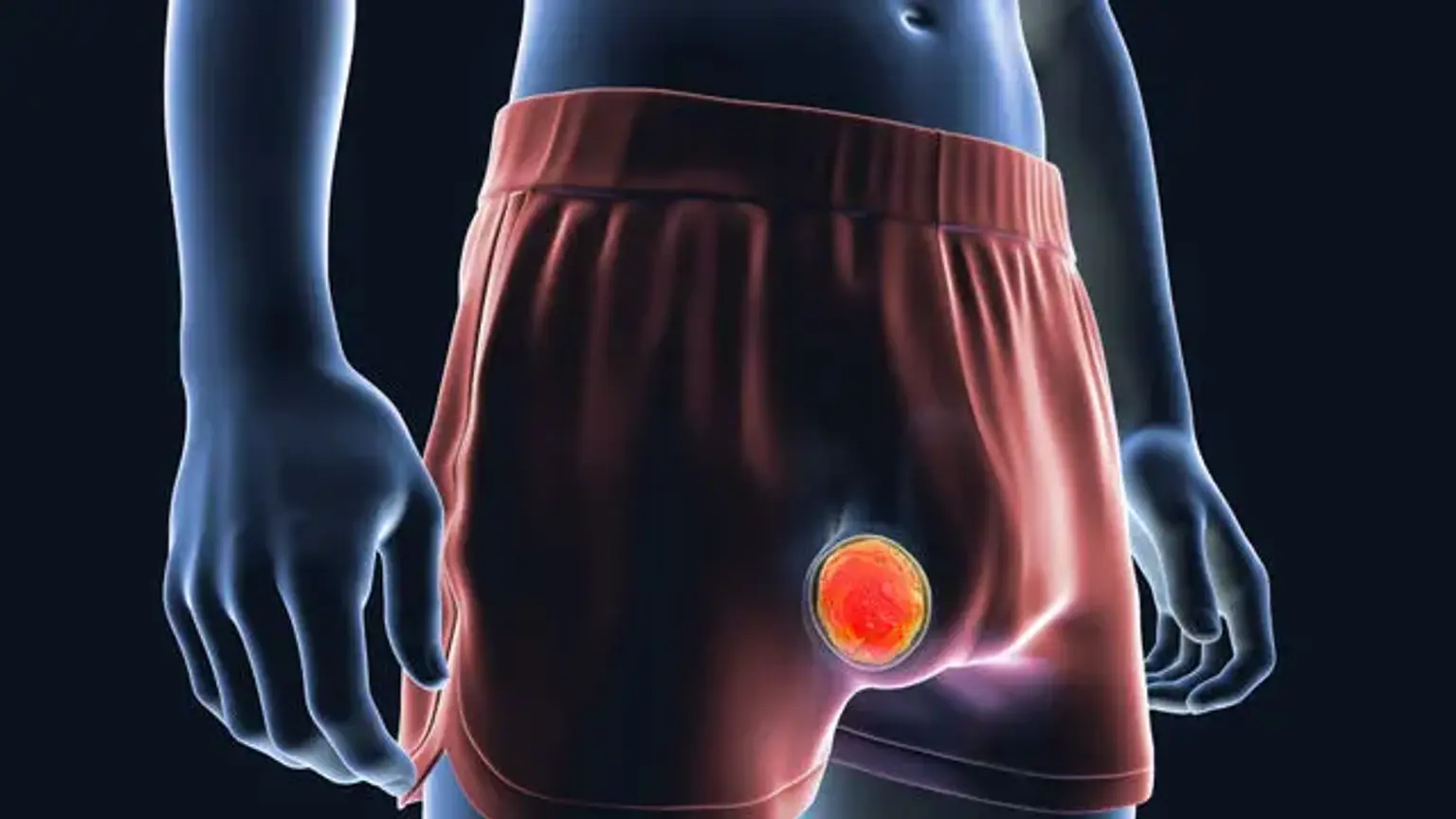
Testicular cancer
Overview
Testicular cancer is the most prevalent malignancy in males aged 15 to 45 years. It is also one of the most common curable cancers. It accounts for 1% of male tumors and 5% of urological malignancies. The prevalence of testicular cancer has been growing in recent years, gaining importance due to the long-term impact both the disease and its treatment can have on a patient's life. Over the last 40 years, the incidence of testicular cancer has more than doubled.
Testicular cancer is caused by a combination of environmental and genetic variables; typical risk factors include cryptorchidism, a family history of testicular cancer, a personal history of testicular cancer in the contralateral testis, age, and ethnicity. The first evaluation consists of a history and physical examination, tumor marker analysis, and scrotal ultrasound.