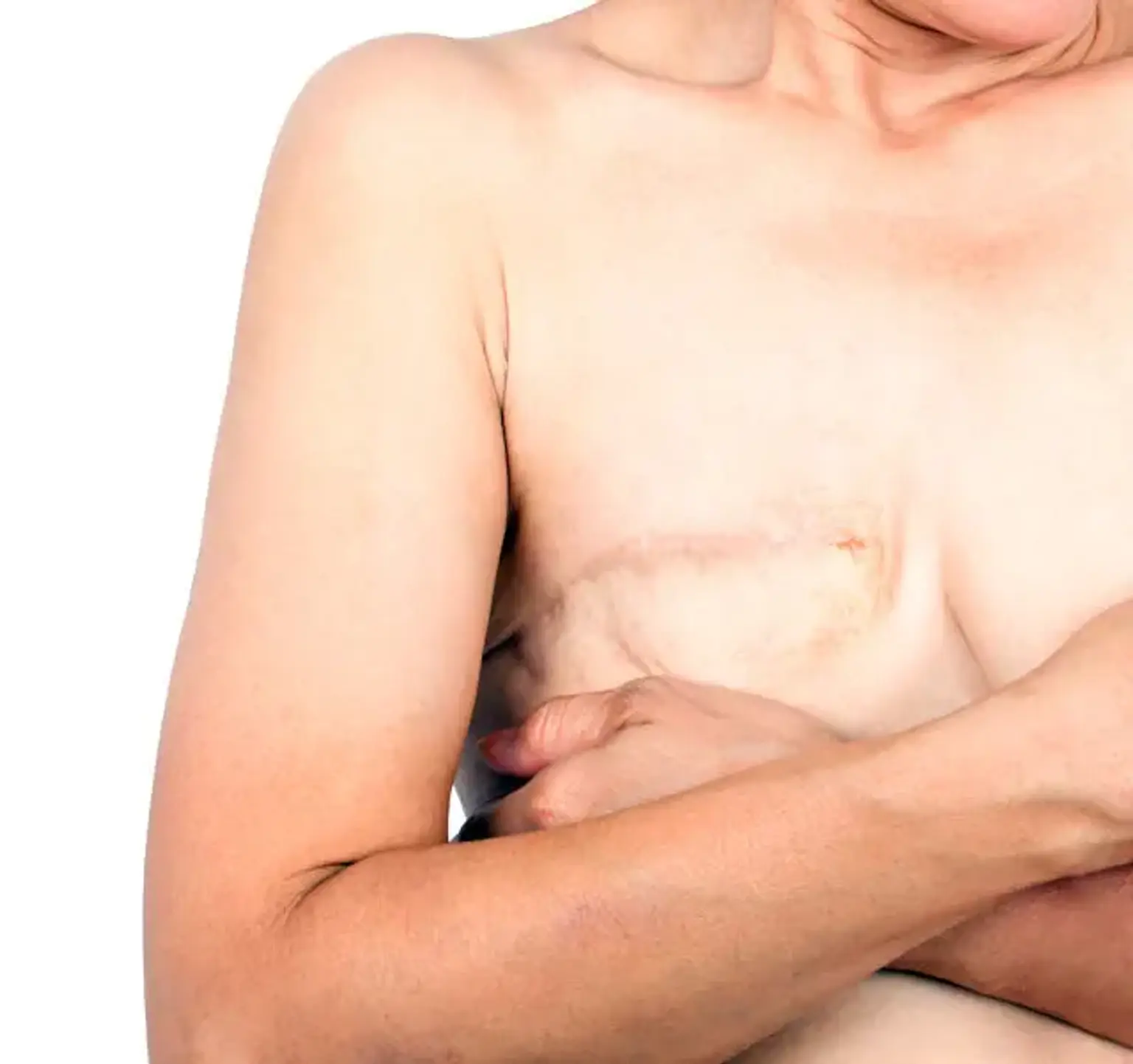
Total Mastectomy
Overview
For patients who are candidates for a preventive mastectomy, a total mastectomy is a successful procedure. This procedure eliminates a greater proportion of breast tissue than subcutaneous mastectomy. Total mastectomy with quick reconstruction has better aesthetic outcomes than subcutaneous mastectomy.