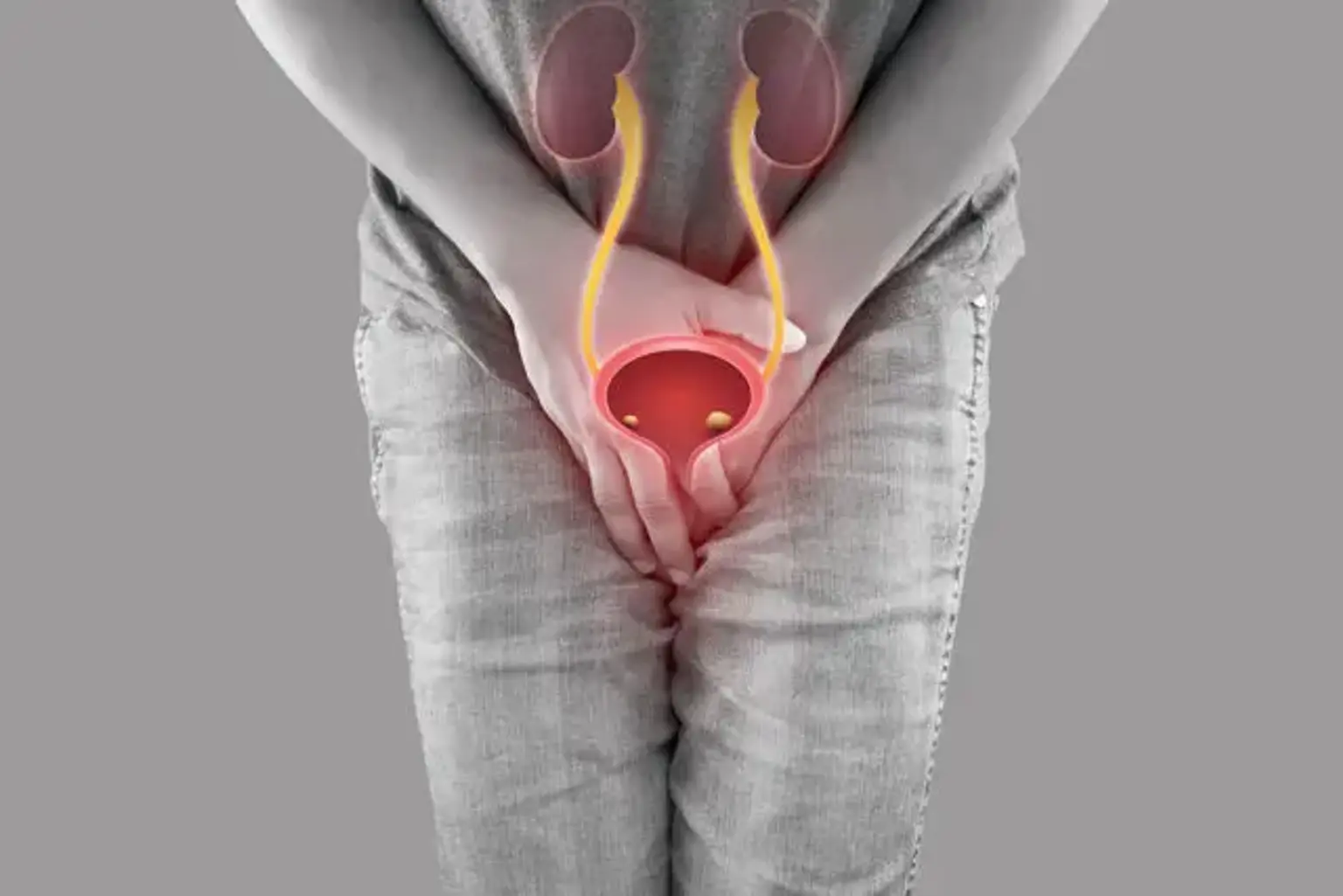
Urethral cancer
Overview
Urethral cancer is a condition in which malignant (cancer) cells originate in the urethral tissues. The urethra is a tube that transports urine from the bladder to the exterior of the body. The urethra is around 112 inches long in women and is located directly above the vagina.