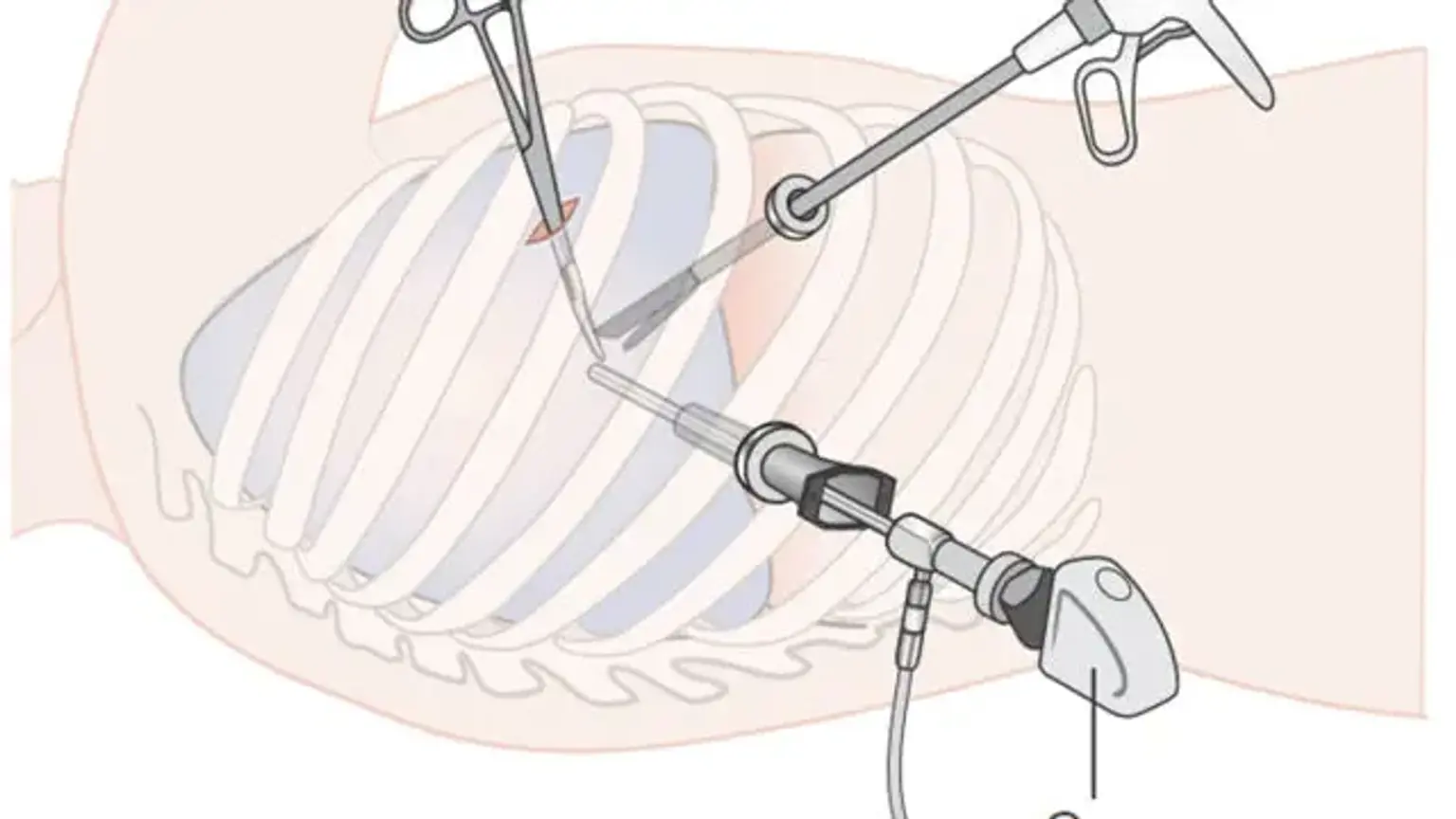
Video-assisted thoracoscopic surgery (VATS)
Overview
Video-assisted thoracoscopic surgery (VATS) is a minimally invasive surgical technique that has made great progress in the detection and treatment of a variety of pulmonary and cardiac problems during the last two decades.
Prior to this technique, the standard treatment for a thoracic pathology was a thoracotomy. Previously, the procedure was often used to evaluate and treat pleural effusions in patients with pulmonary tuberculosis. The development of fiber-optic light was a technical breakthrough that accelerated the advancement of all sorts of minimum access surgery.
The number of VATS surgeries conducted has grown over time as technological advances have made these treatments safer for the elderly and disabled. For example, the vast majority of doctors advocate VATS for lobectomies, which are often done under general anesthesia with One-Lung Ventilation.