Micro Tese (2 testicle)
Overview
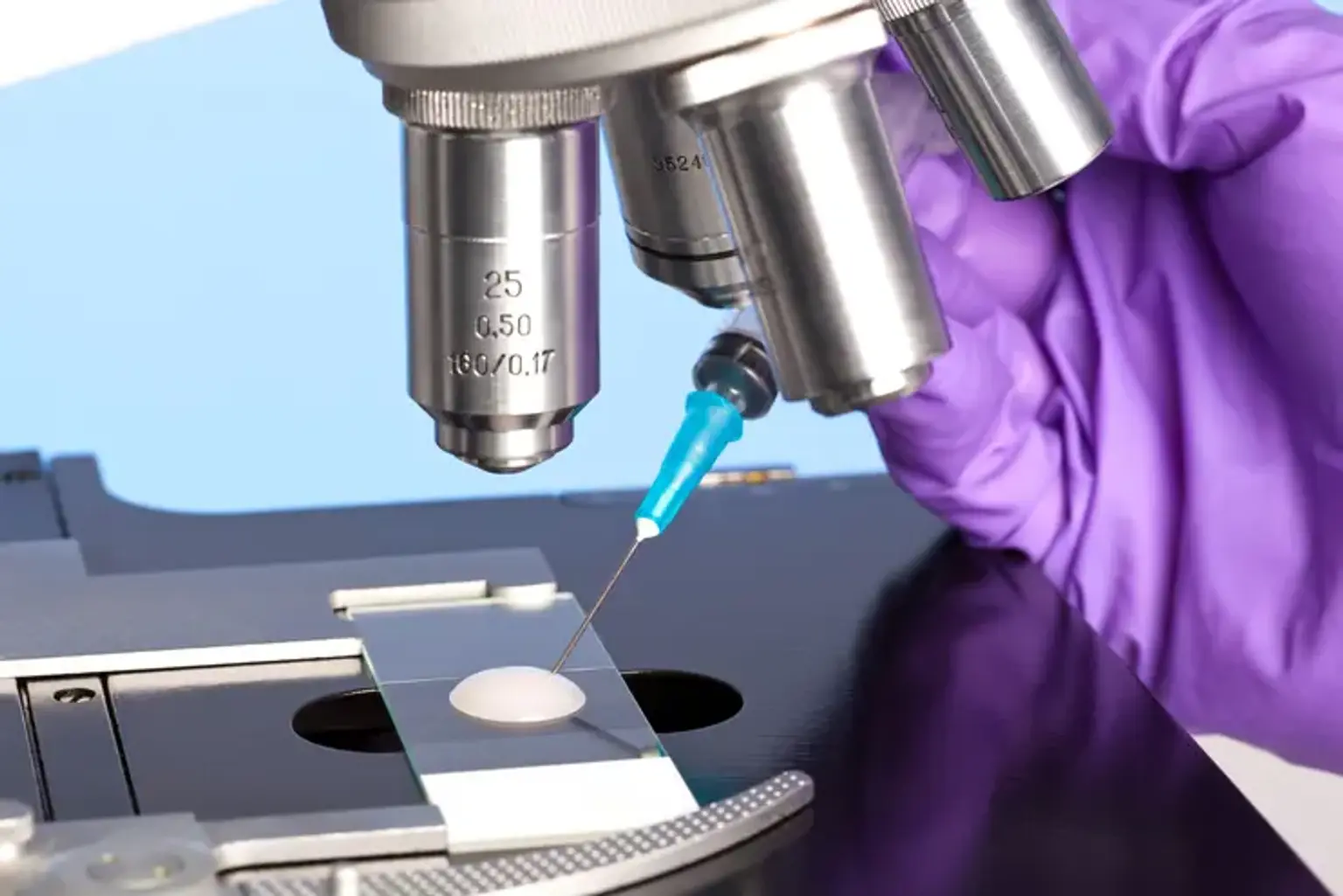
MicroTESE (microscopic testicular sperm extraction) is a method of extracting sperm directly from the testicular tissue of a man's reproductive system. This medical therapy may be administered for reproductive difficulties if a man is unable to create or release enough quality sperm on his own. The testicular tissue is found in the two testes, which are responsible for sperm production. The testes are housed within the scrotum, a little sac behind and under the penis.
The aims of the microTESE process are to collect the highest quality sperm possible, to obtain enough sperm to fertilize a woman's egg, and to limit harm to the reproductive organs.