Neuromuscular Diseases
Overview
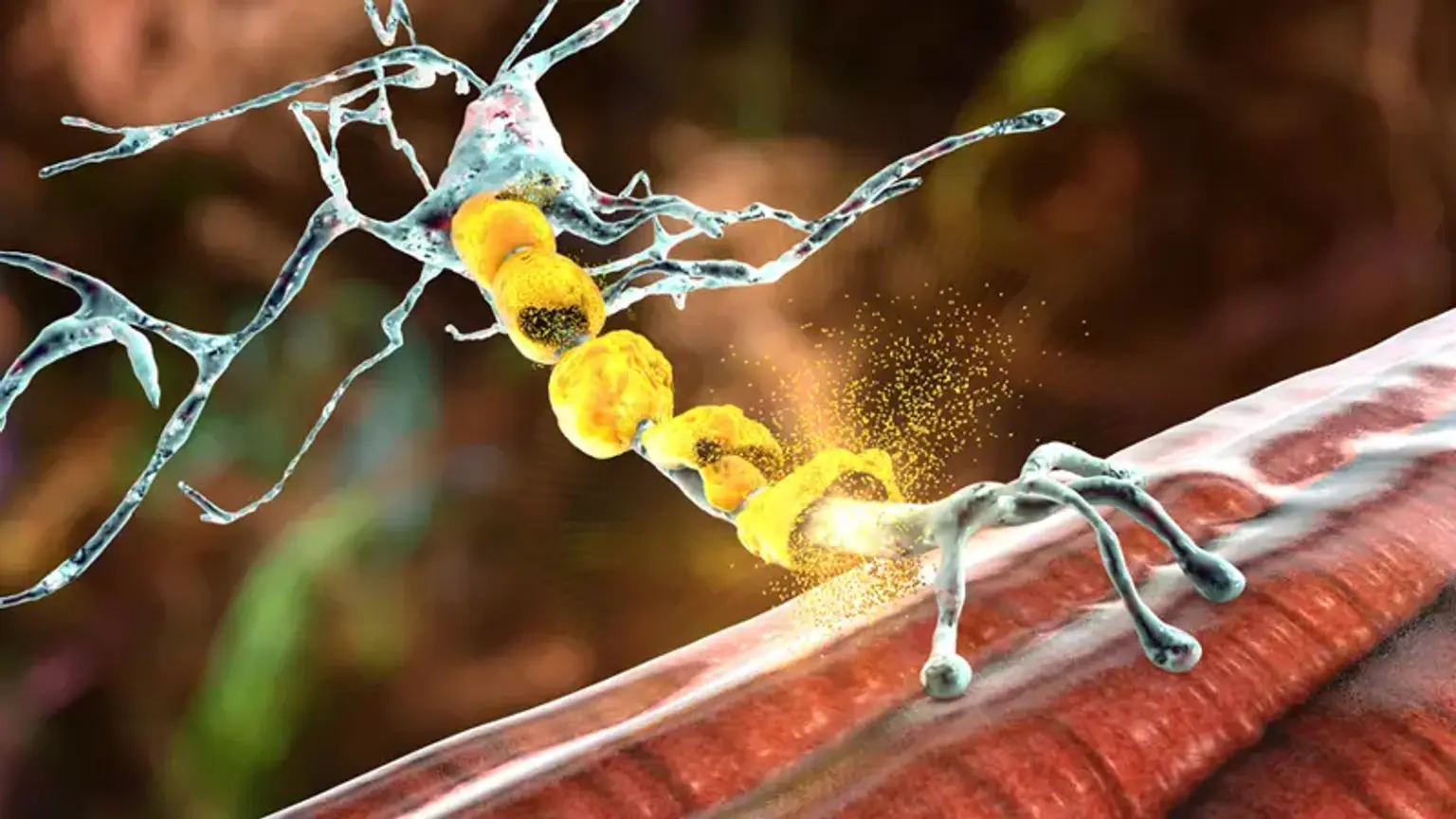
The neuromuscular system is the combination of the neurological system and muscles that work together to allow movement. Through specialized nerves, the brain directs the motions of skeletal (voluntary) muscles. The neuromuscular system is the combination of the neurological system and muscles that work together to allow movement.
A message is transmitted to certain neurons (nerve cells) called higher motor neurons when you desire to move a section of your body. Upper motor neurons have lengthy axons that go into and through the brain, as well as into the spinal cord, where they link with lower motor neurons. Lower motor neurons in the spinal cord deliver their axons directly to the muscle they regulate via nerves in the arms and legs.
There are several illnesses classed as neuromuscular disorders. Neuromuscular diseases induce muscle weakness in the body as a result of a breakdown in communication between the neurological system and the muscles it controls.