Endovascular Therapy
Overview
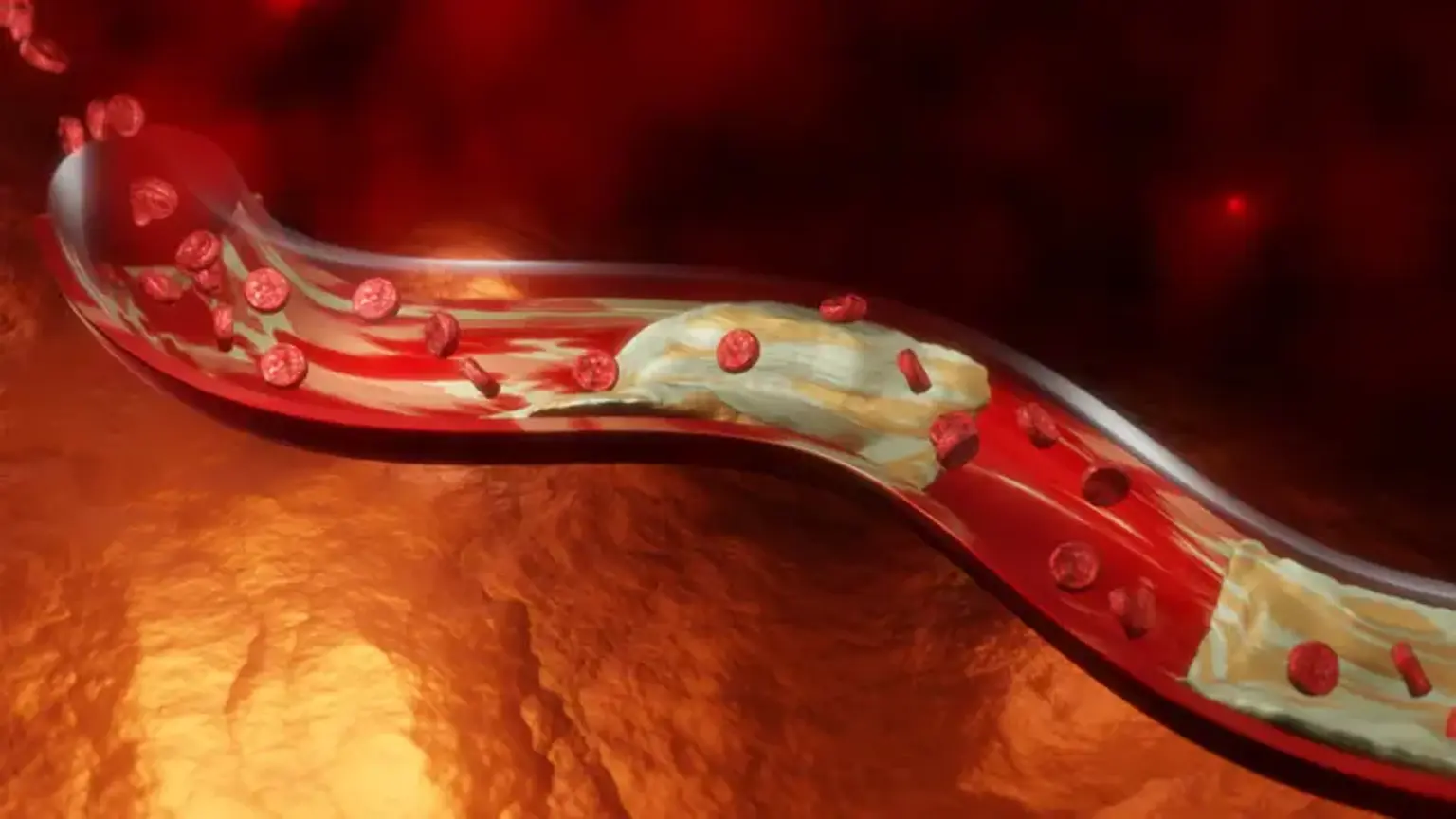
Peripheral artery disease develops when blood arteries thin or get clogged by plaque over time, a condition known as atherosclerosis.
An ischemic stroke happens when a blood artery in the brain becomes blocked or clots. When plaque builds up on the interior wall of an artery, it can cause a blockage or clot. As the cells move through the artery, they adhere to the plaque, causing the blockage or clot (also known as a thrombus) to develop large enough to obstruct normal blood flow. Stroke symptoms include a drooping face, difficulty to lift both arms, and slurred or confused speech.
Rehabilitation and drugs, such as tissue plasminogen activator, blood thinners, and blood pressure medications, can be used to treat stroke. Physical changes (ability to communicate, ability to move, and bowel and bladder issues), relationship changes, legal and financial issues (ability to earn a living, return to work), and less visible changes (emotional changes, fatigue, changes in perception) can all result from a stroke (e.g., vision, sensation, spatial relations, time awareness, unilateral body neglect, visual neglect).