Atrial septal defect (ASD)
Overview
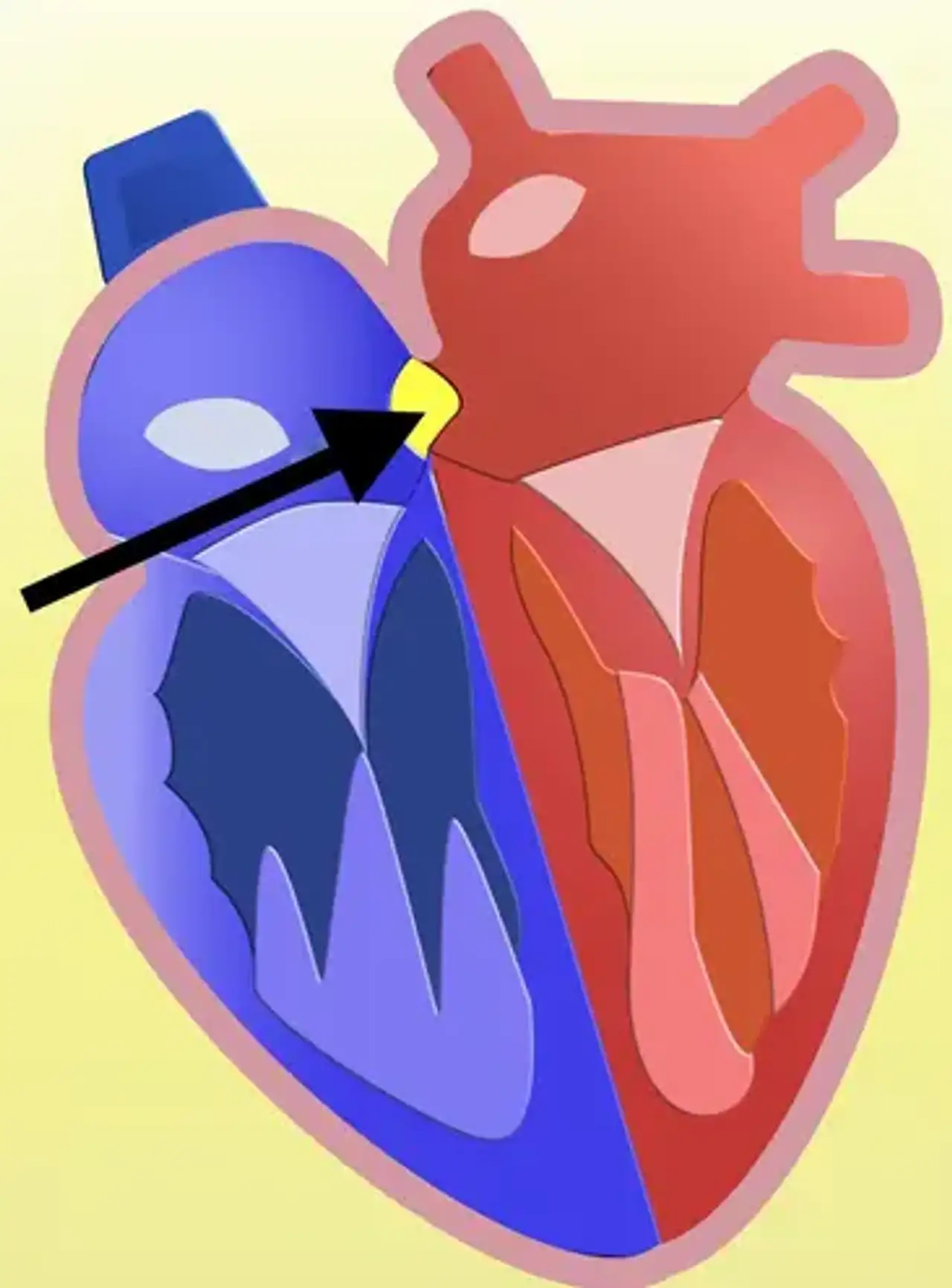
The left side of the heart normally only pumps blood to the body, whereas the right side only pumps blood to the lungs. Blood can pass through the hole from the left upper chamber (left atrium) to the right upper chamber (right atrium) and out into the pulmonary arteries in a kid with ASD.
If the ASD is severe, the excess blood pushed into the lung arteries causes the heart and lungs to work harder, and the pulmonary arteries might gradually deteriorate. If the hole is tiny, there may be no signs or issues.
What is Atrial septal defect (ASD)?
ASD is one of the most prevalent forms of congenital heart abnormalities, affecting around 25% of children. An atrial septal defect arises when the link between the right and left atria is not closed.
It includes both true septal membrane defects and other defects that allow communication between the two atria. From most common to least common, there are five types of atrial septal defects: patent foramen ovale, ostium secundum defect, ostium primum defect, sinus venosus defect, and coronary sinus defect. Small atrial septal abnormalities normally close on their own in youth. Large lesions that do not heal on their own may necessitate percutaneous or surgical intervention to avoid future problems such as stroke, dysrhythmias, and pulmonary hypertension.
Over the last 50 years, the prevalence of congenital cardiac disease and ASDs has grown. Congenital cardiac disease was discovered in less than 1 in 1000 live births in the 1930s. Congenital heart disease has been detected in 9 out of every 1000 live newborns in recent years. Between 1945 and 1949, less than 0.5 live newborns per 1000 were found to have atrial septal abnormalities. According to more current epidemiologic statistics, ASDs occur in 1.6 out of every 1000 live births.
The observed rise in incidence is most likely attributable to advancements in imaging modalities and practitioner training rather than an increase in illness. Several variables, particularly advanced maternal age, have been linked to an increased prevalence of congenital cardiac disease. Interestingly, the diagnostic has shown economic and regional inequalities. Congenital cardiac disease is more frequent in persons with greater earnings in industrialized nations.
Normal anatomy
The typical human heart is in charge of receiving and pumping blood effectively. Because the body's tissues rely on blood to transmit sustenance (oxygen, glucose) and waste products, proper blood flow is critical to health (carbon dioxide). Under normal circumstances, the cardiovascular system (heart, blood vessels) has two distinct circulatory systems: venous and arterial .
Venous (deoxygenated, blue) blood returns to the right atrium from the body. Blood travels from the right atrium into the right ventricle via the tricuspid valve, where it is actively pushed over the pulmonary valve and into the pulmonary arteries. The major pulmonary artery is divided into massive right and left pulmonary arteries, which are further divided into tiny pulmonary arterioles. Numerous pulmonary air-filled sacs are located next to the tiny arterioles. Gases are transferred inside these tiny sacs.
When we breathe out, waste materials from the venous blood enter the air sacs and are eliminated from the pulmonary system (exhale). When we breathe in (inspiration), oxygen enters the lungs and diffuses into the blood via the tiny air sacs. The left atrium receives oxygen-rich (red) blood from the lungs via pulmonary veins.
The right (blue blood) and left (red blood) sides of the heart are separated by muscular and connective tissues. The atrial septum separates the receiving chambers (right and left atria) from the pumping chambers (right and left ventricles). The ventricular septum separates the pumping chambers from the receiving chambers. It is abnormal for the atrial and ventricular septa to remain open in postnatal life.
Atrial septal defect Causes
ASDs are connected with Mendelian inheritance, aneuploidy, transcription mistakes, mutations, and maternal exposures, despite the fact that they arise as single abnormalities. Patients with Down syndrome, Treacher-Collins syndrome, Thrombocytopenia-absent radii syndrome, Turner syndrome, and Noonan syndrome have atrial septal abnormalities; these diseases are caused by Mendelian inheritance. Maternal rubella and drug exposure, such as cocaine and alcohol, can potentially predispose the unborn fetus to ASD.
Atrial septal abnormalities develop in conjunction with other congenital cardiac problems (i.e., ventricular septal defects). Communication between the left and right heart circulations is critical for survival in some patients with congenital heart disease. Patients with tricuspid atresia, transposition of the great vessels, hypoplastic left heart syndrome variants, pulmonic/tricuspid atresia with hypoplastic right heart, transposition of the great vessels, and total anomalous pulmonary venous return require the alternate blood flow provided by an ASD to survive the primary lesion until a more definitive solution is found.
How does the ASD affect my child?
During the fourth week of pregnancy, atrial septation begins. Septation begins with the formation of the main atrial septum (septum primum), which extends caudally from the primitive atrial roof to the endocardial cushions. Mesenchymal cells generated from the embryonic endocardium cover the caudal end of the septum primum.
The septum primum closes and eventually obliterates the ostium primum as it grows toward and attaches to the atrioventricular endocardial cushions. The primitive atrium is divided into right and left atria when the leading edge of the septum primum attaches anteriorly to the atrioventricular cushions. Dorsally, the septum primum joins to the dorsal mesenchymal protrusion. Cell death occurs in the dorsal side of the septum primum when the ostium primum closes, generating the ostium secundum.
The septum secundum grows from the atria's roof to the right of the septum primum. The foramen ovale is formed by the gap established between the septum primum and the septum secundum as it develops caudally and partially covers the ostium secundum. The foramen ovale in the fetus permits oxygen-rich blood to skip the lungs by passing straight from the right atrium to the left atrium. When the infant is born and starts breathing, the shift in pulmonary vascular resistance and subsequent drop in right atrial pressure allows the septum primum to seal the foramen ovale.
As a subclass of the ostium secundum defect, the patent foramen ovale is not a defect of the "true septum." The patent foramen ovale is the most common septal anomaly and occurs when the septum primum and septum secundum do not approximate and close the foramen ovale after the child breathes its first breath. The four forms of atrial septal defects are as follows:
- Ostium secundum defect: This defect arises when the septum primum reabsorbs excessively in the atrium's ceiling or when the septum secundum fails to occlude the ostium secundum. Defects in the osium secundum are linked to pediatric disorders including Noonan, Treacher-Collins, and thrombocytopenia-absent radii syndrome.
- Ostium primum defect: The third most frequent atrial septum defect caused by the septum primum failing to merge with the endocardial cushions. Ostium primum defects cause atrioventricular communications and are best classified as part of the atrioventricular septal defect spectrum.
- Sinus venosus defect: Superior and inferior defects occur, and neither involves the true membranous septum.
- Superior defect: The superior vena cava orifice crosses the atrial septum above the oval fossa (the remains of the foreman ovale) and drains both the left and right atria. The superior sinus venosus defect is frequently associated with a partial abnormal attachment of the right superior pulmonary vein to the superior vena cava.
- Inferior defect: This happens when the orifice of the inferior vena cava takes precedence over both atria. A deficiency in the inferior sinus venosus and an abnormal attachment of the right inferior pulmonary vein to the inferior vena cava can coexist. The inferior defect occurs less frequently than the superior fault.
4. Coronary sinus defect: The coronary sinus is a channel that runs through the groove between the left atrium and left ventricle and collects veins representing the heart muscle's venous return. It generally drains onto the right atrial floor, just above the septal leaflet of the tricuspid valve. A gap or hole in the common wall between the left atrium and the coronary sinus (known as "unroofing") allows contact between the right and left atria.
Signs and Symptoms of ASD
Asymptomatic atrial septal abnormalities are common. A quiet, systolic ejection murmur across the pulmonic region (second intercostal space) is accompanied with a broad, fixed splitting of S2. Many ASDs go misdiagnosed until adulthood, therefore treatment, particularly for significant deficits, is frequently delayed. Large abnormalities that go untreated can lead to exercise intolerance, cardiac dysrhythmias, palpitations, an increased risk of pneumonia, pulmonary hypertension, and death.
Eisenmenger syndrome is an uncommon yet severe consequence of untreated ASDs induced by continuous overflow vascular remodeling (through a left-to-right shunt). Right atrial pressures approach systemic as vascular resistance rises. When right atrial pressures reach systemic pressures, shunt flow reverses. Patients with Eisenmenger syndrome suffer persistent cyanosis, increased pulmonary vascular resistance, exertional dyspnea, syncope, and an increased susceptibility to infection.
People with minor heart abnormalities (less than 5 mm) may not experience any symptoms, but patients with defects ranging from 5 to 10 mm will experience symptoms in their fourth or fifth decade of life. Patients with greater abnormalities present earlier, in their third decade. Patients may complain of dyspnea, weariness, inability to exercise, palpitations, or indications of right-sided heart failure. Preoperatively, around 20% of adult patients develop atrial tachydysrhythmias. Evidence of a stroke or transient ischemic event, particularly after the diagnosis of a peripheral blood clot, should raise concerns for an ASD.
Diagnosis of ASD
Diagnostic imaging is critical in establishing the magnitude of the lesion as well as treatment alternatives. The gold standard imaging modality is a transthoracic echocardiography. A transthoracic echocardiogram can detect the size of the defect, determine the direction of blood flow, identify associated abnormalities (involvement of endocardial cushions and atrial-ventricular valves), examine the heart for structure and function, estimate pulmonary artery pressure, and calculate the pulmonary/systemic flow ratio. A transesophageal echocardiography is a more accurate method for detecting uncommon heart abnormalities.
Although echocardiography is the gold standard for evaluating ASDs, cardiac CT and MRI are also diagnostic modalities. CT and MRI both look at structures around the heart and in the thoracic cavity. Although chest x-ray results are not as diagnostically useful, they do help clinicians monitor clinical conditions by recognizing cardiomegaly and pulmonary artery enlargement. Exercise testing can aid in determining the reversibility of shunt flow and the responsiveness of pulmonary artery hypertension patients to activity. Cardiac catheterization is not recommended in young individuals with modest, uncomplicated ASDs.
Treatment for ASD
In the first year of life, patients with atrial septal defects smaller than 5 mm in size typically have spontaneous closure of the defect. Defects larger than 1cm in length will almost certainly need medical/surgical intervention to close. Control of the aberrant rhythm and anticoagulation is required for patients who come with atrial dysrhythmias; final intervention can occur once the team has gained control of the dysrhythmia.
Adult patients with minor abnormalities and no symptoms of right heart failure should be monitored. Every 2 to 3 years, an echocardiogram is performed to assess the function and anatomy of the right heart. A history of TIA or stroke necessitates more extensive surveillance and, in certain cases, surgical intervention.
Percutaneous and surgical intervention are alternatives for ASD closure. Stroke, a hemodynamically significant shunt bigger than 1.5:1, and signs of systemic oxygen desaturation are all indications for therapy. Percutaneous transcatheter closure is less risky for the patient, but it is only useful for ostium secundum defect closure. Percutaneous transcatheter ASD closure had a 7.2% post-procedural complication risk against a 24% post-surgical complication risk.
Arrhythmias, AV blocks, cardiac erosion, and thromboembolism are all risks linked with percutaneous closure. Small hemodynamically insignificant ASDs, ostium primum defects, sinus venosus defects, coronary sinus defects, and secundum defects with severe pulmonary hypertension are all contraindications to percutaneous closure.
Patients who have atrial septal defects closed percutaneously require antiplatelet treatment for the next 6 months. Women with large ASDs and Eisenmenger Syndrome should avoid pregnancy owing to the dangers of worsening pulmonary artery hypertension and an increased chance of dysrhythmias. Atrial septal defects are surgically closed by inserting a patch over the lesion through an incision in the right atrium.
Finally, atrial septal abnormalities are frequent congenital heart defects that can vary from clinically asymptomatic to those that cause pulmonary hypertension, systemic cyanosis, and vascular consequences such as strokes. The majority of tiny defects resolve on their own during the first year of life; however, substantial defects coupled with considerable systemic to pulmonary shunts and systemic oxygen desaturation necessitate percutaneous or surgical repair.
Adult and Adolescent Management
Adults might be diagnosed with an atrial septal defect for the first time. ASDs come in a variety of sizes. A minor ASD may have no impact on a person's health. Repair is advised if ASDs are significant enough to cause the right heart chambers to enlarge. Secundum ASDs are the most prevalent type. Using a device, they can generally be closed without surgery. Other types of ASDs necessitate surgical intervention. Typically, they are low-risk procedures. Doctors with experience in congenital surgery are essential.
Adults who have had their ASD corrected or closed need to be evaluated on a regular basis. Patients who have had ASD device closure should get an ECHO every five years to look for device problems. Patients who had pulmonary hypertension (high blood pressure in the lungs) before the ASD was closed should continue to see their cardiologist. Adults who have experienced rhythm disorders such as atrial flutter or atrial fibrillation may need to be evaluated on a frequent basis.
What are the Long-term Consequences of ASD?
Potential long-term consequences of atrial defects include:
- Increased blood flow to the right side of the heart (left to right shunting)
- Volume overloading in the right-sided heart chambers (left to right shunting)
- Right ventricular weakening
- Swelling in the veins of the neck, liver, and extremities
- Increased blood flow to the lungs (pulmonary over circulation)
- Pulmonary congestion
- Increased chest infections
- Development of high pressures in the blood vessels in the lungs (pulmonary hypertension)
- Emboli are blood clots that migrate through the bloodstream. Emboli can pass through the atrial septal defect, enter the arterial circulation, and become stuck in a tiny artery of the brain, heart, kidneys, limbs, legs, or other organs, resulting in stroke, heart attack, or organ damage.
- Irregularities in heart rhythm caused by stretched right atrium/ventricle
- Sudden unexpected cardiac death, usually due to arrhythmia
ASD closure is commonly suggested for children and adults to reduce the long-term consequences of ASD (volume and pressure overload, severe arrhythmias) and to avoid embolic events.
Frequently asked questions about ASD
1. What activities can my child do?
Your youngster may not require any particular measures and may be able to engage in usual activities without risk. Your child's pediatric cardiologist may suggest certain activity modifications for a short time following surgery or catheter closure. However, no limitations are normally required following satisfactory recovery from surgery or catheter closure. For a few months following ASD closure, medications to prevent blood clots and infection may be needed.
2. What will my child need in the future?
Depending on the kind of ASD, your child's pediatric cardiologist may assess him or her on a regular basis to search for unusual abnormalities. A pediatric cardiologist must assess the youngster on a frequent basis following surgery to seal an ASD. Long-term prognosis is favorable, and typically no medications, surgery, or catheterization are required.
3. What about preventing endocarditis?
Depending on the kind of ASD, your child's pediatric cardiologist may assess him or her on a regular basis to search for unusual abnormalities. A pediatric cardiologist must assess the youngster on a frequent basis following surgery to seal an ASD. Long-term prognosis is favorable, and typically no medications, surgery, or catheterization are required.
Conclusion
An atrial septal defect (ASD) is a hole in the separating wall (atrial septum) between the right and left upper chambers of the heart. Many youngsters show no symptoms and appear to be healthy. However, if the ASD is big, allowing a substantial flow of blood to pass through to the right side of the heart, the right atrium, right ventricle, and lungs will get overworked, resulting in symptoms. ASD can be corrected surgically in the operating room or through a heart catheterization technique.